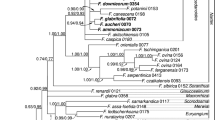
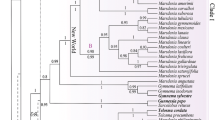
Abstract
Andean orogeny and the ecological changes that followed promoted diversification in plant and animal lineages since the Early Miocene. The angiosperm genus Caiophora (Loasaceae, subfam. Loasoideae) comprises around 50 species that are endemic to South America. These are distributed from southern Ecuador to Central Chile and Argentina. Bee pollination and distribution at low-intermediate elevations probably represent the ancestral condition in the lineage that includes Caiophora and its allied genera. The majority of Caiophora species grow at high elevations in the Andes, where some depend on vertebrate pollination. Previous studies did not resolve phylogenetic relationships within Caiophora, which precluded the dating of the origin and divergence of this group. We used markers of one nuclear (ITS) and one plastid region (trnSGCU-trnGUUC) to solve phylogenetic relationships among 19 Caiophora species (including different accessions). We also included 10 species of the allied genera Blumenbachia and Loasa. Aosa rostrata and Xylopodia klaprothioides were used as outgroups. Phylogenetic reconstruction strongly supports the monophyly of Caiophora, and although several clades within this genus are poorly supported, our study yielded a better infra-generic resolution than previous studies. The origin of Caiophora is dated to the Early-Middle Miocene and can be related to the uplift of the Cordilleras Frontal and Principal and to Pacific marine transgressions. According to our estimations, Caiophora began to diversify during the Middle-Late Miocene and this unfolding proceeded eastwards during the Pliocene and the Pleistocene, in parallel to the uplift of different Andean mountain ranges.


Similar content being viewed by others
References
Ackermann, M. (2012). Studies on systematics, morphology and taxonomy of Caiophora and reproductive biology of Loasaceae and Mimulus (Phrymaceae). PhD Thesis. Free University of Berlin, Germany.
Ackermann, M., & Weigend, M. (2006). Nectar, floral morphology and pollination syndrome in Loasaceae subfam. Loasoideae (Cornales). Annals of Botany, 98, 503–514.
Ackermann, M., Achatz, M., & Weigend, M. (2008). Hybridization and crossability in Caiophora (Loasaceae, subfam. Loasoideae): are interfertile species and inbred populations results of a recent radiation? American Journal of Botany, 95, 1109–1121.
Altshuler, D. L., Dudley, R., & McGuire, J. A. (2004). Resolution of a paradox: hummingbird flight at high elevation does not come without a cost. Proceedings of the National Academy of Sciences of the United States of America, 101, 17731–17736.
Antonelli, A., & Sanmartín, I. (2011). Why are there so many plant species in the Neotropics? Taxon, 60, 403–414.
Antonelli, A., Nylander, J. A., Persson, C., & Sanmartín, I. (2009). Tracing the impact of the Andean uplift on Neotropical plant evolution. Proceedings of the National Academy of Sciences, 106, 9749–9754.
Armijo, R., Lacassin, R., Coudurier-Curveur, A., & Carrizo, D. (2015). Coupled tectonic evolution of Andean orogeny and global climate. Earth-Science Reviews, 143, 1–35.
Arroyo, M. T. K., Primack, R., & Armesto, J. J. (1985). Community studies in pollination ecology in the high temperate Andes of Central Chile. I. Pollination mechanism and altitudinal variation. American Journal of Botany, 69, 82–97.
Baranzelli, M. C., Johnson, L. J., Cosacov, A., & Sérsic, A. N. (2014). Historical and ecological divergence among populations of Monttea chilensis (Plantaginaceae), an endemic endangered shrub bordering the Atacama Desert, Chile. Evolutionary Ecology, 28, 751–774.
Barnes, J. B., & Ehlers, T. A. (2009). End member models for Andean Plateau uplift. Earth-Science Reviews, 97, 105–132.
Bechis, F., Encinas, A., Concheyro, A., Litvak, V. D., Aguirre-Urreta, B., & Ramos, V. A. (2014). New age constraints for the Cenozoic marine transgressions of northwestern Patagonia, Argentina (41°-43° S): paleogeographic and tectonic implications. Journal of South American Earth Sciences, 52, 72–93.
Bryson Jr., R. W., García-Vázquez, U. O., & Riddle, B. R. (2012). Relative roles of Neogene vicariance and Quaternary climate change on the historical diversification of bunchgrass lizards (Sceloporus scalaris group) in Mexico. Molecular Phylogenetics and Evolution, 62, 447–457.
Cavides-Vidal, E., Bozinovic, F., & Rosenmann, M. (1987). Thermal freedom of Graomys griseoflavus in a seasonal environment. Comparative Biochemistry and Physiology Part A: Physiology, 87, 257–259.
Chaves, J. A., Weir, J. T., & Smith, T. B. (2011). Diversification in Adelomyia hummingbirds follows Andean uplift. Molecular Ecology, 20, 4564–4576.
Clapperton, C. H. (1983). The glaciation of the Andes. Quaternary Science Reviews, 2, 83–155.
Cocucci, A. A., & Sérsic, A. N. (1998). Evidence of rodent pollination in Cajophora coronata (Loasaceae). Plant Systematics and Evolution, 211, 113–128.
Cosacov, A., Sérsic, A. N., & Sosa, V. (2010). Multiple periglacial refugia in the Patagonian steppe and post-glacial colonization of the Andes: the phylogeography of Calceolaria polyrhiza. Journal of Biogeography, 37, 1463–1477.
Cruden, R. W. (1972). Pollinators in high-elevation ecosystems: relative effectiveness of birds and bees. Science, 176, 1439–1440.
Donato, M., Posadas, P., Miranda-Esquivel, D. R., Jaureguizar, E. O., & Cladera, G. (2003). Historical biogeography of the Andean region: evidence from Listroderina (Coleoptera: Curculionidae: Rhytirrhinini) in the context of the South American geobiotic scenario. Biological Journal of the Linnean Society, 80, 339–352.
Drummond, C. S., Eastwood, R. J., Miotto, S. T. S., & Hughes, C. E. (2012a). Multiple continental radiations and correlates of diversification in Lupinus (Leguminosae): testing for key innovations with incomplete taxon sampling. Systematic Biology, 61, 443–460.
Drummond, A. J., Suchard, M. A., Xie, D., & Rambaut, A. (2012b). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution, 29, 1969–1973.
Echavarria, L., Hernndez, R., Allmendinger, R., & Reynolds, J. (2003). Subandean thrust and fold belt of northwestern Argentina: geometry and timing of the Andean evolution. AAPG Bulletin, 87, 965–985.
Felsenstein, J. (1984). Distance methods for inferring phylogenies: a justification. Evolution, 38, 16–24.
Fenster, C. B., Armbruster, W. S., Wilson, P., Dudash, M. R., & Thomson, J. D. (2004). Pollination syndrome and floral specialization. Annual Review of Ecology, Evolution and Systematics, 25, 375–403.
Gaiero, D. M., Simonella, L., Gassó, S., Gili, S., Stein, A. F., Sosa, P., Becchio, R., Arce, J., & Marelli, H. (2013). Ground/satellite observations and atmospheric modeling of dust storms originating in the high Puna-Altiplano deserts (South America): implications for the interpretation of paleo-climatic archives. Journal of Geophysical Research: Atmospheres, 118, 3817–3831.
Gamble, T., Simons, A. M., Colli, G. R., & Vitt, L. J. (2008). Tertiary climate change and the diversification of the Amazonian gecko genus Gonatodes (Sphaerodactylidae, Squamata). Molecular Phylogenetics and Evolution, 46, 269–277.
Garzione, C. N., Molnar, P., Libarkin, J. C., & MacFadden, B. J. (2006). Rapid late Miocene rise of the Bolivian Altiplano. Evidence from removal of mantle lithosphere. Earth and Planetary Science Letters, 241, 543–556.
Giambiagi, L. B., & Ramos, V. A. (2002). Structural evolution of the Andes in a transitional zone between flat and normal subduction (33°30′–33°45′S), Argentina and Chile. Journal of South American Earth Studies, 15, 101–116.
Graham, A., Gregory-Wodzicki, K. M., & Wright, K. L. (2001). Studies in Neotropical Paleobotany. XV. A Mio-Pliocene palynoflora from the Eastern Cordillera, Bolivia: implications for the uplift history of the Central Andes. American Journal of Botany, 88, 1545–1557.
Hamilton, M. B. (1999). Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Molecular Ecology, 8, 521–523.
Hoiss, B., Krauss, J., Potts, S. G., Roberts, S., & Steffan-Dewenter, I. (2012). Altitude acts as an environmental filter on phylogenetic composition, traits and diversity in bee communities. Proceedings of the Royal Society B-Biological Sciences, 279, 4447–4456.
Hoke, G. D., & Garzione, C. N. (2008). Paleosurfaces, paleoelevation, and the mechanisms for the late Miocene topographic development of the Altiplano plateau. Earth and Planetary Science Letters, 271, 192–201.
Hufford, L., McMahon, M. M., Sherwood, A. M., Reeves, G., & Chase, M. W. (2003). The major clades of Loasaceae: phylogenetic analysis using the plastid matK and trnL-trnF regions. American Journal of Botany, 90, 1215–1228.
Hufford, L., McMahon, M. M., O’Quinn, R., & Poston, M. E. (2005). A phylogenetic analysis of Loasaceae, subfamily Loasoideae based on plastid DNA sequences. International Journal of Plant Sciences, 166, 289–300.
Hughes, C., & Eastwood, R. (2006). Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences of the United States of America, 103, 10334–10339.
Jobb, G., Haeseler, A. V., & Strimmer, K. (2004). TREEFINDER: a powerful graphical analysis environment for molecular phylogenetics. BMC Evolutionary Biology. doi:10.1186/1471-2148-4-18.
Katoh, K., & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30, 772–780.
Lamb, S., Hoke, L., Kennan, L., & Dewey, J. (1997). Cenozoic evolution of the Central Andes in Bolivia and northern Chile. Geological Society, London, Special Publications, 121, 237–264.
Levina, M., Horton, B. K., Fuentes, F., & Stockli, D. F. (2014). Cenozoic sedimentation and exhumation of the foreland basin system preserved in the Precordillera thrust belt (31–32°S), southern central Andes, Argentina. Tectonics, 33, 1659–1680.
Luebert, F., & Weigend, M. (2014). Phylogenetic insights into Andean plant diversification. Frontiers in Ecology and Evolution. doi:10.3389/fevo.2014.00027.
Martinez, J. J., Ferro, L. I., Mollerach, L. I., & Barquez, R. M. (2012). The phylogenetic relationships of the Andean swamp rat genus Neotomys (Rodentia, Cricetidae, Sigmodontinae) based on mitochondrial and nuclear markers. Acta Theriologica, 57, 277–287.
Martini, M.A., Strelin, J.A., Kaplan, M.R., & Schaefer, J.M. (2012). Glacial and periglacial geomorphology and chronology around the Nevado de Chañi (Cordillera Oriental of Jujuy): implication for past climate in NW Argentina. Resource document. AGU Fall Meeting Abstracts. http://adsabs.harvard.edu/abs/2012AGUFMEP53C1053M. Accessed 13 Oct 2016.
McGuire, J. A., Witt, C. C., Remsen Jr., J. V., Corl, A., Daniel, L., Rabosky, D. L., Altshuler, D. L., & Dudley, R. (2014). Molecular phylogenetics and the diversification of hummingbirds. Current Biology, 24, 910–916.
Meng, H. H., & Zhang, M. L. (2013). Diversification of plant species in arid Northwest China: species-level phylogeographical history of Lagochilus Bunge ex Bentham (Lamiaceae). Molecular Phylogenetics and Evolution, 68, 398–409.
Mercer, J. H., & Sutter, J. (1982). Late Miocene—earliest Pliocene glaciation in Southern Argentina: implications for global ice-sheet history. Palaeogeography, Palaeoclimatology, Palaeoecology, 38, 185–206.
Minelli, A., & Fusco, G. (2012). On the evolutionary developmental biology of speciation. Evolutionary Biology, 39, 242–254.
Moré, M., Cocucci, A. A., & Sérsic, A. N. (2015). Phylogeny and floral trait evolution in Jaborosa (Solanaceae). Taxon, 64, 523–534.
Muschick, M., Indermaur, A., & Salzburger, W. (2012). Convergent evolution within an adaptive radiation of cichlid fishes. Current Biology, 22, 2362–2368.
Mutke, J., Jacobs, R., Meyers, K., Henning, T., & Weigend, M. (2014). Diversity patterns of selected Andean plant groups correspond to topography and habitat dynamics, not orogeny. Frontiers in Genetics. doi:10.3389/fgene.2014.00351.
Müller, J., Müller, K., Quandt, D., & Neinhuis, C. (2010). PhyDe-Phylogenetic Data Editor. Program distributed by the author. Available at: http://www.phyde.de/index.html.
Nespolo, R. F., Opazo, J. C., & Rosenmann, F. B. (1999). Thermal acclimation, maximum metabolic rate, and nonshivering thermogenesis of Phyllotis xanthopygus (Rodentia) in the Andes Mountains. Journal of Mammalogy, 80, 742–748.
Nielsen, R. (2002). Mapping mutations on phylogenies. Systematic Biology, 51, 729–739.
Nylin, S., Slove, J., & Janz, N. (2014). Host plant utilization, host range oscillations and diversification in Nymphalid butterflies: a phylogenetic investigation. Evolution, 68, 105–124.
Palazzesi, L., Gottschling, M., Barreda, V., & Weigend, M. (2012). First Miocene fossils of Vivianiaceae shed new light on the phylogeny, divergence times, and historical biogeography of Geraniales. Botanical Journal of the Linnean Society, 107, 67–85.
Perez, F., Arroyo, M. T. K., Medel, R., & Hershkovitz, M. A. (2006). Ancestral reconstruction of flower morphology and pollination systems in Schizanthus (Solanaceae). American Journal of Botany, 93, 1029–1038.
Ramos, V. A., Cristallini, E. O., & Pérez, D. J. (2002). The Pampean flat-slab of the Central Andes. Journal of South American Earth Studies, 15, 59–78.
Revell, L. J. (2012). Phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223.
Revell, L. J. (2014). Graphical methods for visualizing comparative data on phylogenies. In L. Z. Garamszegi (Ed.), Modern phylogenetic comparative methods and their application in evolutionary biology (pp. 77–103). Berlin, Heidelberg: Springer.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Rubioldo, D., Seggiaro, R., Gallardo, E., Disalvo, A., Sanchez, M., Turel, A., Ramallo, E., Sandruss, A., & Godeas, M. (2001). Hoja Geológica 2366-II / 2166-IV, La Quiaca. Geología y Provincias de Jujuy y Salta. Instituto de Recursos Minerales, Servicio Geológico Minero Argentino. Boletín 246, p. Buenos Aires.
Rutschmann, F. (2006). Molecular dating of phylogenetic trees: a brief review of current methods that estimate divergence times. Diversity and Distributions, 12, 35–48.
Sanderson, M. J. (1997). A nonparametric approach to estimating divergence times in the absence of rate constancy. Molecular Biology and Evolution, 14, 1218–1231.
Schenk, J. J., & Hufford, L. (2010). Effects of substitution models on divergence time estimates: simulations and an empirical study of model uncertainty using Cornales. Systematic Botany, 35, 578–592.
Sérsic, A., Cosacov, A., Cocucci, A. A., Johnson, L. A., Pozner, R., Avila, L. J., Sites Jr., J. W., & Morando, M. (2011). Emerging phylogeographical patterns of plants and terrestial vertebrates from Patagonia. Biological Journal of the Linnean Society, 103, 475–494.
Smith, S. D., & Baum, D. A. (2006). Phylogenetics of the florally diverse Andean clade Iochrominae (Solanaceae). American Journal of Botany, 93, 1140–1153.
Smith, S. D., Ané, C., & Baum, D. A. (2008). The role of pollinator shifts in the floral diversification of Iochroma (Solanaceae). Evolution, 62, 793–806.
Solà, E., Sluys, R., Gritzalis, K., & Riutort, M. (2013). Fluvial basin history in the northeastern Mediterranean region underlies dispersal and speciation patterns in the genus Dugesia (Platyhelminthes, Tricladida, Dugesiidae). Molecular Phylogenetics and Evolution, 66, 887–888.
Starck, D., & Anzótegui, L. M. (2001). The late Miocene climatic change—persistence of a climatic signal through the orogenic stratigraphic record in northwestern Argentina. Journal of South American Earth Sciences, 14, 763–774.
Stech, M., Veldman, S., & Larraín, J. (2013). Molecular species delimitation in the Racomitrium canescens Complex (Grimmiaceae) and implications for DNA barcoding of species complexes in mosses. PloS One. doi:10.1371/journal.pone.0053134.
Strelin, M. M., Benitez-Vieyra, S., Ackermann, M., & Cocucci, A. (2016a). Flower reshaping in the transition to hummingbird pollination in Loasaceae, subfam. Loasoideae despite absence of corolla tubes or spurs. Evolutionary Ecology, 30, 401–407.
Strelin, M. M., Benitez-Vieyra, S., Fornoni, J., Klingenberg, C. P., & Cocucci, A. A. (2016b). Exploring the ontogenetic scaling hypothesis during the diversification of pollination syndromes in Caiophora (Loasaceae, subfam. Loasoideae). Annals of Botany, 117, 937–947.
Swofford, D.L. (2003). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
Särkinen, T., Pennington, R. T., Lavin, M., Simons, M. F., & Hughes, C. E. (2012). Evolutionary islands in the Andes: persistence and isolation explain high endemism in Andean dry tropical forests. Journal of Biogeography, 39, 884–900.
Weigend, M., & Gottschling, M. (2006). Evolution of funnel-revolver flowers and ornithophily in Nasa (Loasaceae). Plant Biology, 8, 120–142.
Weigend, M., Gottschling, M., Hoot, S., & Ackermann, M. (2004). A preliminary phylogeny of Loasaceae subfam. Loasoideae (Angiospermae: Cornales) based on trnL(UAA) sequence data, with consequences for systematics and historical biogeography. Organisms Diversity & Evolution, 4, 73–90.
Weigend, M., Gröger, A., & Ackermann, M. (2005). The seeds of Loasaceae subfam. Loasoideae (Cornales) II: seed morphology of “South Andean Loasas” (Loasa, Caiophora, Scyphanthus and Blumenbachia). Flora-Morphology, Distribution, Functional Ecology of Plants, 200, 569–591.
Weigend, M., Ackermann, M., & Henning, T. (2010). Reloading the revolver- male fitness as a simple explanation for complex reward partitioning in Nasa macrothyrsa (Loasaceae, Cornales). Biological Journal of the Linnean Society, 100, 124–131.
Wen, J., & Zimmer, E. (1996). Phylogeny and biogeography of Panax L (the ginseng genus, Araliaceae): inferences from ITS sequences of nuclear ribosomal RNA. Molecular Phylogenetics and Evolution, 6, 167–177.
West-Eberhard, M. J. (2005). Developmental plasticity and the origin of species differences. Proceedings of the National Academy of Sciences, 102, 6543–6549.
Xia, X., & Xie, Z. (2001). DAMBE: software package for data analysis in molecular biology and evolution. Journal of Heredity, 92, 371–373.
Xia, X., Xie, Z., Salemi, M., Chen, L., & Wang, Y. (2003). An index of substitution saturation and its application. Molecular Phylogenetics and Evolution, 26, 1–7.
Yang, Z., & Rannala, B. (2012). Molecular phylogenetics: principles and practice. Nature Reviews Genetics, 13, 303–314.
Zech, J., Zech, R., Kubik, P. W., & Veit, H. (2009). Glacier and climate reconstruction at Tres Lagunas, NW Argentina, based on 10 Be surface exposure dating and lake sediment analyses. Palaeogeography, Palaeoclimatology, Palaeoecology, 284, 180–190.
Zuloaga, F.O., Morrone, O., Belgrano, M.J., Marticorena, C., & Marchesi, E. (2008). Catálogo de las plantas vasculares del Cono Sur. Monogr. Syst. Bot. Missouri Bot. Gard, 107(1–3), i–xcvi, 1–3348.
Acknowledgements
We thank Marcela Moré and Cristina Acosta (IMBIV, CONICET, Universidad Nacional de Córdoba, Argentina); Romina Vidal-Russel (INIBIOMA, CONICET, Universidad Nacional del Comahue); Jorge Strelin, Mateo Martini and Diego Gaiero (CICTERRA, Universidad Nacional de Córdoba, Instituto Antártico Argentino); Matías Ghiglione (Instituto de Estudios Andinos, CONICET, Universidad Nacional de Buenos Aires, Argentina); and the two anonymous reviewers for contributing with their comments and suggestions to the quality of this manuscript. We thank Laura Gatica for editing the English of this manuscript and Maximilian Weigend (Nees Institut für Biodiversität der Pflanzen, Rheinische Friedrich-Wilhelms-Universität, Bonn, Germany) for providing material, funds and data for this study. M.S. has a scholarship from the National Scientific and Technical Research Council (CONICET).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s13127-016-0318-y.
Electronic supplementary material
Table S1
(XLS 28 kb)
Table S2
(XLS 48 kb)
Table S3
(DOC 95 kb)
Appendix S1
(XML 89 kb)
Table S4
(DOCX 15 kb)
Fig. S1.
Substitution saturation plot. Relationship between observed (uncorrected) and estimated (corrected) genetic distances for a F84 model of nucleotide substitution. S: transitions and V: transversions. Linear relationship indicates no saturation. (JPEG 52 kb)
Fig. S2.
Phylogenetic trees based on concatenated ITS/ trnSGCU-trnGUUC markers obtained with: a) MP; b) NJ; c) ML; d) BI methods. (PDF 26 kb)
Fig. S3.
Phylogenetic trees based on ITS markers obtained with: a) MP; b) NJ; c) ML; d) BI methods. (PDF 27 kb)
Fig. S4.
Phylogenetic trees based on trnSGCU-trnGUUC markers obtained with: a) MP; b) NJ; c) ML; d) BI methods. (PDF 25 kb)
Fig. S5.
Consensus phylogenetic trees for: a) ITS; b) trnSGCU-trnGUUC; c) ITS/ trnSGCU-trnGUUC for the four reconstruction methods: maximum parsimony (MP), neighbour joining (NJ), maximum likelihood (ML), and bayesian inference (BI). (PDF 34 kb)
Rights and permissions
About this article
Cite this article
Strelin, M.M., Arroyo, J.I., Fliesswasser, S. et al. Diversification of Caiophora (Loasaceae subfam. Loasoideae) during the uplift of the Central Andes. Org Divers Evol 17, 29–41 (2017). https://doi.org/10.1007/s13127-016-0312-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-016-0312-4