Abstract
Parkin is an ubiquitin‐E3 ligase that is involved in cellular mitophagy and was recently shown to contribute to controlling adipose tissue thermogenic plasticity. We found that Parkin expression is induced in brown (BAT) and white (WAT) adipose tissues of aged mice. We determined the potential role of Parkin in the aging-associated decline in the thermogenic capacity of adipose tissues by analyzing subcutaneous WAT, interscapular BAT, and systemic metabolic and physiological parameters in young (5 month-old) and aged (16 month-old) mice with targeted invalidation of the Parkin (Park2) gene, and their wild-type littermates. Our data indicate that suppression of Parkin prevented adipose accretion, increased energy expenditure and improved the systemic metabolic derangements, such as insulin resistance, seen in aged mice. This was associated with maintenance of browning and reduction of the age-associated induction of inflammation in subcutaneous WAT. BAT in aged mice was much less affected by Parkin gene invalidation. Such protection was associated with a dramatic prevention of the age-associated induction of fibroblast growth factor-21 (FGF21) levels in aged Parkin-invalidated mice. This was associated with a parallel reduction in FGF21 gene expression in adipose tissues and liver in aged Parkin-invalidated mice. Additionally, Parkin invalidation prevented the protein down-regulation of β-Klotho (a key co-receptor mediating FGF21 responsiveness in tissues) in aged adipose tissues. We conclude that Parkin down-regulation leads to improved systemic metabolism in aged mice, in association with maintenance of adipose tissue browning and FGF21 system functionality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thermogenic capacity of brown adipose tissue (BAT) and beige adipose tissue resulting from the “browning” of white fat (WAT) declines with aging in humans and experimental rodent models [37]. This decline is considered to contribute to the propensity of elderly individuals to develop metabolic diseases, from overweight to type 2 diabetes and dyslipidemia. The mechanisms leading to the age-associated functional decline in thermogenic adipose tissue are poorly understood despite the obvious biomedical interest in preventing this shift.
Fibroblast growth factor 21 (FGF21) is a member of the endocrine FGF subfamily that is produced primarily in the liver and adipose tissues. Treatment with FGF21 ameliorates metabolic disorders such as insulin resistance, dyslipidemia and obesity in mice [6, 19], and treatment of human volunteers with FGF21 analogs has shown beneficial effects on metabolic disorders ranging from obesity and type 2 diabetes to non-alcoholic liver diseases [31]. The capacity of FGF21 to activate BAT thermogenesis and promote the “beiging” of WAT [7, 11, 17] is considered to contribute to its beneficial metabolic effects. The actions of FGF21 are mediated through interactions with FGF receptors on the cell surface and require the coreceptor, β-klotho [27]. In fact, the expression of β-klotho determines the specific responsiveness of cells to FGF21. Transgenic overexpression of FGF21 in mice, leading to high levels of circulating FGF21, was reported to extend the lifespan [36], prompting researchers to propose that FGF21 is a pro-longevity endocrine factor. Consistent with this, FGF21 was reported to critically mediate the beneficial effects on lifespan elicited by dietary protein restriction [16]. On the other hand, FGF21 levels in blood increase with age in rodents and humans [12, 15]. This could seem somewhat paradoxical considering the healthy metabolic effects expected from high FGF21 levels and the tendency of metabolic alterations to appear with age. It has been hypothesized that the age-related increases in FGF21 are related to the appearance of an FGF21-resistant state, as has been proposed to occur in metabolic diseases such as obesity and type 2 diabetes [10, 29]. However, recent data from aged humans are not compatible with this proposal [33], and low levels of FGF21 have been related to healthy aging in centenarians [30].
Recent work revealed that Parkin contributes to controlling BAT activity and the browning of WAT. Parkin is an ubiquitin‐E3 ligase that acts as a key component of the cellular machinery for mitophagy and apoptosis. The name comes from the identification of mutations in the Parkin-encoding gene as being responsible for early-onset forms of Parkinson disease, a neurodegenerative condition that is usually associated with aging [8]. The actions of Parkin are reported to be reciprocally related to the thermogenic activity of adipose tissues. Parkin is required for the adaptive down-regulation of BAT thermogenic activity [1, 27] and the induction of “whitening” in beige adipose tissue [20, 32] in response to cessation of environmental thermal challenges, likely through its role in promoting the degradation of components of the mitochondrial thermogenic machinery.
Here we report that Parkin levels in adipose tissue increase with aging which led us to hypothesize that Parkin could be involved in the aging-associated down-regulation of the thermogenic properties of adipose tissues. Therefore, we studied the impact of Parkin invalidation on thermogenic adipose tissues in aged mice and characterized the key alterations elicited by Parkin invalidation on the age-induced decline in WAT browning and FGF21 homeostasis.
Materials and methods
Mouse studies
For studies on changes in Parkin expression across aging, male C57/BL6 mice, obtained from Envigo, were used. For studies on the effects of Parkin gene invalidation, Parkin‐KO (B6.129S4‐Park2tm1Shn/J) mice were obtained from Jackson Laboratories and wild‐type (WT) littermates were used as controls. Mice were housed at 22 °C under a 12-h light cycle with free access to water. Mice were fed a standard diet (3.1 kcal/g, 24% protein, 58% carbohydrates, 18% fat; Teklad, Envigo). Male mice were studied at ages of 5 months (adult) and 16 months (aged). The volume of consumed oxygen and CO2 released was determined using an Oxylet system and analyzed with the Oxylet Metabolism 3.0 software (Harvard Apparatus). Oxygen consumption measurements, calculations of metabolic efficiency, glucose tolerance tests (GTT), and insulin tolerance tests (ITT) were performed in the 3-week period before sacrifice. For GTT, glucose in aqueous solution was administered intraperitoneally (2.5 g glucose/kg) to overnight-fasted mice, blood was obtained from the tail 0, 15, 30, 60, 90, 120, and 150 min after glucose injection, and glycemia was measured. For ITT, insulin (Actrapid; Novo Nordisk Pharma) in saline solution was administered intraperitoneally (0.75 UI/kg) to 4-h-starved mice, and glycemia in blood obtained from the tail was measured 0, 15, 30, 45, and 60 min after glucose injection. Areas under the curve were calculated from individual curves of glycemia changes after GTT and ITT.
After sacrifice, interscapular BAT (iBAT), inguinal WAT (iWAT), epididymal adipose tissue (eWAT), liver, heart and tibia were dissected, and blood was collected in heparinized tubes to obtain plasma. All experiments were performed in accordance with European Community Council Directive 86/609/EEC, and the experiments and numbers of animals to be used were approved by the Institutional Animal Care and Use Committee of the University of Barcelona.
Analytical procedures for blood and plasma samples
Glucose and triglyceride levels in blood were measured using Accutrend Technology (Roche Diagnostics). Plasma FGF21 was quantified using an enzyme-linked immunosorbent assay (ELISA) (RD291108200R, BioVendor). Plasma insulin and leptin levels were quantified using a Multiplex system (MADKMAG‐HK, Merck Millipore).
Optical microscopy
For hematoxylin and eosin (H&E) staining, tissue samples were fixed overnight in 4% paraformaldehyde, paraffin‐embedded and processed in a microtome. Tissue sections of 4 μm thickness were mounted onto glass slides, dewaxed, rehydrated, stained with H&E, dehydrated again in a graded ethanol/xylene series and sealed with DPX and a coverslip, according to standard procedures. Adipocyte size was analyzed in sections of inguinal adipose tissue; digital images were captured using an Olympus DP70 digital camera (Olympus) attached to a bright-field light microscope (Nikon Eclipse 90i Upright Microscope). At least four fields of view were analyzed for each sample (n = 3 samples per genotype per timepoint). All images were acquired under the same conditions at 200 × magnification. Adipocyte sizes were measured using the open-source imaging program, Fiji (a distribution of ImageJ), with the semi-automated plug-in, Adiposoft.
RNA isolation and quantitative real‐time PCR
RNA was extracted from adipose tissues and liver using a NucleoSpin RNA column kit (Macherey‐Nagel). Reverse transcription of 0.5 μg of total RNA in a total reaction volume of 20 µL was performed using a high-capacity complementary DNA (cDNA) kit. Samples were systematically checked for null amplification in the absence of reverse transcriptase. qRT-PCR was performed using the appropriate TaqMan probes (Supplemental Table 1); each 25 µL reaction mixture contained 1 µL cDNA, 12.5 µL TaqMan Universal PCR Master Mix (Thermo Fisher Scientific) and 250 nM probes. The cDNA level of each gene of interest was normalized to that of a housekeeping reference gene (Ppia mRNA). The comparative CT (2−ΔCt) method was applied for normalization, according to the manufacturer’s instructions. Main results were checked using the 18S rRNA as a second independent housekeeping gene.
Western blotting
Tissue extracts were prepared by homogenization in a buffer consisting of 20 mM Tris–HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA, 1% Triton X‐100, a protease inhibitor cocktail (Roche Diagnostics), 2 mM sodium orthovanadate, and 10 mM β‐glycerophosphate. The total protein content was measured using a Pierce™ BCA Protein Assay Kit (Fisher Scientific). Equal amounts of protein (30 µg) were separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12% or 15% gradient gels and electrotransferred onto Immobilon‐P PVDF (polyvinylidene difluoride) membranes (GE Healthcare). The membranes were incubated with primary antibodies specific for Parkin (2132, Cell Signaling Technology), β-klotho (ab76356, Abcam) and tyrosine hydroxylase (ab152, Merck Millipore), and then with horseradish peroxidase (HRP)‐conjugated anti‐mouse IgG (170‐6516, Bio‐Rad) or anti‐rabbit IgG (sc‐2004, Santa Cruz), as appropriate. Signals were detected using a chemiluminescence‐HRP substrate (WBKLS0100, Merck). Membranes were stained with Ponceau to normalize the amount of protein loaded. Densitometric analyses of digitalized images were performed using the Fiji software (ImageJ).
Statistical analysis
Statistical significance was assessed using the two‐tailed unpaired Student's t‐test, one-way ANOVA or two‐way ANOVA followed by Tukey's post-hoc test, all of which were applied using GraphPad statistical software (GraphPad Software). Discrepancies among standard deviations of experimental groups were analyzed with the F‐test. Welch's correction was applied whenever unequal variances were detected. Exact numbers of replicates are shown in each Figure legend. Outliers were detected using Grubbs’ test and removed prior to analysis of significance. Statistical significance was set at P < 0.05, and the underlying assumptions for validity were assessed for all tests. Data are expressed as means ± standard error of the mean (SEM).
Results
-
1.
Parkin expression is up-regulated in aged brown and white adipose tissues
Parkin gene expression was determined in iWAT and BAT across aging in mice. Parkin mRNA levels increased progressively from 3 to 16 months of age (Fig. 1A). Parkin protein levels were up-regulated in iWAT and BAT from aged mice (16-month-old) relative to young (5-month-old) mice (Fig. 1B).
-
2.
Parkin gene invalidation reduces age-dependent adiposity and associated metabolic derangements
Gene expression and protein levels of Parkin in subcutenous white and brown adipose tissues upon aging. (A) Gene expression levels of Park2 (Parkin gene) in inguinal white adipose tissue (iWAT) and brown adipose tissue (BAT) in mice at 3, 5 and 16 months of age. (B) Representative immunoblot (top) and quantification of Parkin protein levels (bottom) in iWAT and BAT from young (5 month-old) versus aged (16 month-old) mice. N = 3–9. The bars represent means ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001 when comparing young versus aged mice)
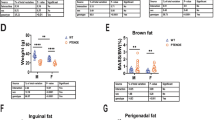
To determine the role of Parkin in the aging of adipose tissues, we analyzed Parkin-KO mice in comparison with wild-type littermates in young adult (5-month-old) and aged (16-month-old) mice (Table 1). At 5 months of age, Parkin-KO mice showed a mild and non-significant trend toward decreased body weight and adipose depot mass compared to wild-type littermates. At 16 months of age, wild-type mice had significantly increased body weight, mostly due to fat accretion (massive enlargement of iWAT and eWAT) relative to young wild-type mice. These age-dependent changes were much weaker in 16-month-old Parkin-KO mice, which showed significantly lower body weight, massive reductions in the amounts of iWAT, eWAT, and iBAT, and even a reduction in liver weight, compared to the corresponding wild-type mice. These changes did not reflect any notable alteration in developmental growth, as evidenced by the lack of difference in tibia length between wild-type and Parkin-KO mice at 16 months of age.
Aged Parkin-KO mice showed systemic metabolic changes relative to aged wild-type mice (Table 1). Wild-type mice at 16 months of age showed higher glycemia, much higher insulin levels than 5-month-old wild-type mice, impaired glucose tolerance and reduced insulin sensitivity in the insulin tolerance test, indicating the appearance of insulin resistance with aging. Most of these alterations were prevented in aged Parkin-KO mice, which did not show basal hyperglycemia or hyperinsulinemia, showed improved insulin sensitivity test relative to aged wild-type mice, although the glucose tolerance was not improved. Triglyceride levels were unaltered in relation to age or genotype, whereas leptin levels were markedly increased at 16 months of age relative to 5 months of age; this alteration was more pronounced in wild-type mice than in Parkin-KO mice, likely reflecting the observed changes in the sizes of WAT depots.
We also observed significantly reduced food intake in 16 month-old Parkin-KO mice relative to wild-type mice. Calculation of metabolic efficiency indicated that the body weight accretion per ingested calorie was significantly lower in 16 month-old Parkin-KO mice than in wild-type mice, indicating that the reduced adiposity in aged Parkin-KO mice is likely to involve enhanced energy expenditure as an additional process contributing to the reduction in body weight. Accordingly, we found significantly higher levels of oxygen consumption, indicative of enhanced energy expenditure, in 16-month-old Parkin-KO mice relative to the corresponding wild-type mice (Table 1), whereas respiratory quotient was not altered by Parkin invalidation at any age. When oxygen consumption data were corrected per body weight, the impaired oxygen consumption associated with aging in wild-type mice was largely prevented in aged Parkin-KO mice.
-
3.
Parkin invalidation partially prevents the age-dependent decline in WAT browning but does not alter BAT thermogenesis decline.
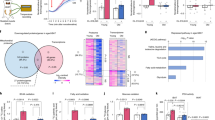
Analysis of the microscopic morphology of iWAT revealed only minor differences between Parkin-KO and wild-type mice at 5 months of age. At 16 months of age, in contrast, white adipocytes were much smaller in iWAT from Parkin-KO mice compared to that of wild-type mice (Fig. 2A), revealing that Parkin invalidation protects mice from age-associated adipocyte hypertrophy. Gene expression analysis showed that wild-type mice experienced declines in the expression levels of marker genes for adipose browning, e.g. Ucp1 (uncoupling protein 1), Dio2 (type II iodothyronine 5’-deiodinase), Cox7a1 (cytochrome c oxidase subunit 7A1), with aging, but this was totally or partially prevented in Parkin-KO mice. Expression of Ucp1 and Pgc1a (PPARγ-coactivator-1α) were higher in iWAT from aged Parkin-KO mice relative to aged wild-type mice (Fig. 2B). The marked up-regulation in leptin gene expression due to aging was also prevented in Parkin-KO mice. The expression levels of genes related to inflammatory pathways, such as Ccl2 (encoding chemokine (C–C motif) ligand 2) and Nos2 (encoding nitric oxide synthase 2), were up-regulated in WAT from aged wild-type mice but not aged Parkin-KO mice (Fig. 2B). In contrast, the expression levels of genes involved in lipid metabolism, oxidative stress, ER stress and autophagy did not reveal relevant changes due to Parkin invalidation in aged mice (Supplemental Table 2). Moreover, the protein levels of tyrosine hydroxylase (TH), an indicator of the extent of sympathetic innervation at adipose tissue, were also reduced with aging in wild-type mice but not in Parkin-KO mice (Fig. 2C). Overall, these data indicate that the extent of WAT browning declines with aging, but Parkin gene invalidation prevents this decline. Moreover, WAT from aged Parkin-KO mice show a healthier phenotype associated with a leaner condition, as evidenced by reduced inflammation.
Adiposity and metabolic functionality markers in white adipose tissue from wild-type and Parkin knock-out young and aged mice. (A) Histological representative images (left) and quantification of white adipocytes area (right) from young (5 month-old) versus aged (16 month-old) wild-type and Parkin-KO mice. (B) Quantification of mRNA levels of markers related to browning (Ucp1, Dio2, Pgc1a, Cox7a1), adipose tissue function (Pparg, Lep, Adipoq), and inflammation (Tnfa, Ccl2, Nos2) in white adipose tissue from young versus aged wild-type and Parkin-KO mice. (C) Quantification and representative image of immunoblots for tyrosine hydroxylase protein in white adipose tissue samples from young versus aged wild-type and Parkin-KO mice. N = 3–7. The bars represent means ± SEM (*p < 0.05, ***p < 0.001 when comparing wild-type versus Parkin-KO mice; #p < 0.05, ##p < 0.01, ###p < 0.001 when comparing young versus aged mice)
Analyses of microscopic morphology and gene expression patterns failed to reveal marked changes caused by Parkin invalidation in BAT from 16-month-old mice (Supplemental Fig. 1A). The expression of marker genes for thermogenic activity (e.g. Ucp1, Pgc1a) declined with aging similarly in wild-type and Parkin-KO mice (Supplemental Fig. 1B). The protein levels of TH were also similarly reduced in BAT from 16-month-old wild-type and Parkin-KO mice relative to 5-month-old wild-type and Parkin-KO mice (Supplemental Fig. 1C). Similarly unaltered gene expression pattern was found in the mRNA levels of genes encoding components of lipid metabolism, oxidative stress, ER stress, and autophagy in Parkin-KO mice at 5 or 16 months of age, relative to wild-type (Supplemental Table 3).
-
4.
Parkin gene invalidation suppresses the aging-dependent inductions of FGF21 mRNA and protein levels while preventing the age-induced decline of β-klotho in adipose tissues.
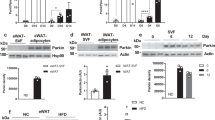
Our endocrine profiling of Parkin-KO mice revealed that the plasma levels of FGF21 were markedly induced in aged wild-type mice but not Parkin-KO mice (Fig. 3A). Consistent with this, analysis of FGF21 gene expression in iWAT revealed that there was an age-dependent induction of Fgf21 mRNA levels in wild-type mice but not Parkin-KO mice (Fig. 3B). Similarly, levels of Fgf21 mRNA remained low in BAT from aged Parkin-KO mice. As the liver critically contributes to FGF21 systemic levels, we analyzed the hepatic expression of FGF21. We found a similar significant reduction in hepatic Fgf21 mRNA levels in 16-month-old Parkin-KO mice relative to 16-month-old wild-type mice. Gene expression analysis of liver from 5-month-old and 16 month-old wild-type and Parkin-KO mice did not reveal significant changes due to Parkin gene invalidation for transcripts encoding components of gluconeogenesis, lipid metabolism, oxidative stress, and ER stress at any age (Supplemental Table 4).
Analysis of FGF21 levels, FGF21 and β-klotho expression in white and brown adipose tissues, and liver, from young versus aged wild-type and Parkin-KO mice. (A) FGF21 circulating levels. (B) Quantification of mRNA levels of Fgf21 in adipose tissues and liver. (C) Quantification of mRNA levels of Klb in adipose tissues and liver. (D) Quantification (bottom) and representative image of immunoblots (top) for β-klotho in brown and white adipose tissues from young versus aged wild-type and Parkin-KO mice. The bars represent means ± SEM. N = 3–8. (*p < 0.05, **p < 0.01, ***p < 0.001 when comparing wild-type versus Parkin-KO mice; #p < 0.05, ##p < 0.01, ###p < 0.001 when comparing young versus aged mice)
We further analyzed the expression levels of the Klb, encoding the co-receptor β-klotho, which is key in providing cells with specific responsiveness to FGF21. For the Klb mRNA, we found that aging was associated with significant down-regulation in BAT, a non-significant trend to reduction in iWAT, and no change in liver (Fig. 3C). Parkin gene invalidation did not significantly modify the mRNA expression of Klb in adipose tissues from aged mice, although there was an increasing trend. However, at the protein level, β-klotho was significantly down-regulated with aging in BAT and iWAT in wild-type but not Parkin-KO mice; the β-klotho protein levels were significantly higher in aged Parkin-KO mice than compared to aged wild-type mice (Fig. 3D). The mRNA expression levels of Fgfr1, encoding FGF receptor-1, were unaltered due to the genotype or age in iWAT (Supplemental Table 2) whereas it was somewhat lower in aged BAT with no difference between Parkin-KO and wid-type mice (Supplemental Table 3).
Discussion
In the present study, we found that Parkin invalidation prevents the increased adiposity and metabolic derangements associated with aging in mice. This is associated with maintenance of the browning of subcutaneous adipose tissue and suppression of the age-induced up-regulation of FGF21.
Our findings that Parkin invalidation has positive effects on metabolism and adiposity in aged mice are consistent with the results of previous studies in young mice challenged with thermogenic stimuli. These prior studies indicated that Parkin, as a component of the mitophagy machinery, contributes to repressing BAT function and promoting the “whitening” of beige adipose tissue [1, 20, 28, 32]. The relatively positive effects of Parkin suppression on aging may appear surprising at first glance, given that the preservation of active mitophagy is important for healthy aging [2]. Our data did not suggest that loss of Parkin impaired mitochondrial function in adipose tissues; this could reflect the action of allostatic processes, which often occur in loss-of-function models in which the targeting of a single component of a biological process (here, mitophagy) elicits intense compensatory processes that overcome any potential derangement due to the invalidation of a single actor. During the preparation of this article, it was reported [24] that Parkin gene invalidation at adipose tissues also leads to prevention of age-associated adiposity, which is consistent with our current findings. On the other hand, Parkin suppression in aged mice resulted in a reduction in food intake, which could indirectly favor a healthier metabolic status and play a role in the preservation of WAT browning. Notably, we also found that Parkin invalidation in aged mice affected preferentially the browning of WAT rather than BAT activity. There are multiple previous reports indicating a higher responsiveness of WAT browning rather than BAT thermogenic activity in experimental models challenging adaptive thermogenesis [26]. This has been attributed to a particular relevance of the regulation of WAT browning in modulating thermogenic adaptation to some metabolic conditions [26, 35] and, perhaps, to the existence of distinct non-UCP1-mediated thermogenic processes in WAT [4].
In addition to global beneficial effects on adipose and insulin resistance, the most marked effect found for Parkin invalidation across aging was suppression of the age-related induction of FGF21, which was noted at the gene expression level in adipose tissues and liver, as well as for systemic FGF21 levels. The age-related increase in FGF21 levels has been noted in humans [15] and rodent models [12], but its biological significance is not fully understood. Given that FGF21 has been proposed as an anti-aging factor [33] and is known to have beneficial effects on metabolism and adiposity [13], its age-related increase might be viewed as a mechanism that attempts to minimize aging-associated derangements. However, the results from studies performed in populations of elderly individuals indicate that there is an inverse association between FGF21 levels and longevity [30]. On the other hand, the age-associated increase in FGF21 levels is reminiscent of that occurring in obesity, suggesting that aging may be associated with a progressive loss of FGF21 responsiveness in tissues, giving rise to a reactive FGF21 up-regulation in a so-called “FGF21 resistance” scenario [10]. This possibility would be consistent with the observation of age-related down-regulation of β-klotho, the key factor for FGF21 responsiveness in adipose tissues [25], suggesting a scenario wherein sensitivity to FGF21 decreases with age in mice. However, the concept of FGF21-resistance is controversial [22] and recent data do not support the idea that an FGF21-resistant state is acquired with age, at least in humans [33]. In any case, the prevention of age-related increases in FGF21 should be viewed as a beneficial metabolic effect of Parkin invalidation. Further research is needed to elucidate the mechanisms, whether direct or indirect, by which the lack of Parkin leads to the down-regulation of FGF21. Considering that mitochondrial oxidative stress and/or endoplasmic reticulum (ER) stress are known to induce FGF21 synthesis in adipose tissues [3, 18], the FGF21 down-regulation observed in Parkin-KO mice is consistent with a lack of mitochondrial dysfunction in these mice, as shown by unaltered expression of marker genes for mitochondrial oxidative stress ROS and ER stress in Parkin-KO mice at any age.
Our data indicate that Parkin invalidation prevents aging-associated β-klotho down-regulation and FGF21 up-regulation. The mechanisms by which lack of Parkin prevents the aging-associated repression of β-klotho (and, accordingly, may preserve FGF21 sensitivity) deserve further research. Pro-inflammatory signals are major repressors of β-klotho expression in adipose tissues [9], and our current data indicate that pro-inflammatory cytokines are down-regulated in WAT from aged Parkin-KO mice; this, therefore, may underlie the observed maintenance of β-klotho levels. Moreover, it cannot be ruled out that preserved levels of β-klotho could maintain the sensitivity to FGF21 and constitute a primary event elicited by Parkin invalidation that reduces age-associated FGF21 secretion. Considering the important role of FGF21 in the browning of subcutaneous WAT, the maintenance of some extent of browning in Parkin-KO mice may also be secondary to maintenance of FGF21 signaling. In any case, the mechanisms by which lack of Parkin prevents the aging-associated repression of β-klotho (and, accordingly, may preserve FGF21 sensitivity) deserve further research. Our findings on FGF21 alterations in aging-associated adipose thermogenic decline do not rule out the potential role of other regulatory factors of adipose browning. The number of potential effectors of adaptive WAT browning is growingly extensive, among them thyroid hormones, bile acids and several peptidic endocrine actors [21,22,23], so further research will be needed to identify their potential role in the response to Parkin invalidation. Moreover, the use of a whole-body Parkin KO model in our study and the signs of altered eating behavior also suggest that centrally-mediated mechanisms may contribute to the observed alterations in adipose plasticity in aged mice. Parkin is widely expressed in brain, including hypothalamus [14] and some reports point to a relevant role of Parkin in the mitophagy processes in hypothalamic regions [34], key to the control of energy balance and thermogenic adipose tissue plasticity [5]. Further research will be needed to explore this possibility and their interplay with the FGF21 system.
Conclusions
Collectively, the present data highlight the beneficial metabolic effects that, along with WAT browning, occur with aging in Parkin-KO mice. The effects of Parkin on the FGF21 system may be relevant to the systemic beneficial effects that Parkin suppression can have on aging.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Cairó M, Campderrós L, Gavaldà-Navarro A, Cereijo R, Delgado-Anglés A, Quesada-López T et al (2019) Parkin controls brown adipose tissue plasticity in response to adaptive thermogenesis. EMBO Rep 20:e46832. https://doi.org/10.15252/embr.201846832
Chen G, Kroemer G, Kepp O (2020) Mitophagy: an emerging role in aging and age-associated diseases. Front Cell Dev Biol 8:200. https://doi.org/10.3389/fcell.2020.00200
Choi MJ, Jung SB, Lee SE, Kang SG, Lee JH, Ryu MJ et al (2020) An adipocyte-specific defect in oxidative phosphorylation increases systemic energy expenditure and protects against diet-induced obesity in mouse models. Diabetologia 63:837–852. https://doi.org/10.1007/s00125-019-05082-7
Chouchani ET, Kazak L, Spiegelman BM (2019) New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab 8:27–37. https://doi.org/10.1016/j.cmet.2018.11.002
Contreras C, Nogueiras R, Diéguez C, Rahmouni K, López M (2017) Traveling from the hypothalamus to the adipose tissue: the thermogenic pathway. Redox Biol 12:854–863. https://doi.org/10.1016/j.redox.2017.04.019
Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y et al (2008) Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149:6018–6027. https://doi.org/10.1210/en.2008-0816
Cuevas-Ramos D, Mehta R, Aguilar-Salinas CA (2019) Fibroblast growth factor 21 and browning of white adipose tissue. Front Physiol 10:37. https://doi.org/10.3389/fphys.2019.00037
Deng H, Wang P, Jankovic J (2018) The genetics of Parkinson disease. Ageing Res Rev 42:72–85. https://doi.org/10.1016/j.arr.2017.12.007
Díaz-Delfín J, Hondares E, Iglesias R, Giralt M, Caelles C, Villarroya F (2012) TNF-α represses β-Klotho expression and impairs FGF21 action in adipose cells: involvement of JNK1 in the FGF21 pathway. Endocrinology 153:4238–4245. https://doi.org/10.1210/en.2012-1193
Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E (2010) Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59:2781–2789. https://doi.org/10.2337/db10-0193
Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F et al (2012) FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26:271–281. https://doi.org/10.1101/gad.177857.111
Fujii N, Uta S, Kobayashi M, Sato T, Okita N, Higami Y (2019) Impact of aging and caloric restriction on fibroblast growth factor 21 signaling in rat white adipose tissue. Exp Gerontol 118:55–64. https://doi.org/10.1016/j.exger.2019.01.001
Giralt M, Gavaldà-Navarro A, Villarroya F (2015) Fibroblast growth factor-21, energy balance and obesity. Mol Cell Endocrinol 418(Pt 1):66–73. https://doi.org/10.1016/j.mce.2015.09.018
Gu WJ, Abbas N, Lagunes MZ, Parent A, Pradier L, Bohme GA et al (2000) Cloning of rat parkin cDNA and distribution of parkin in rat brain. J Neurochem 74(4):1773–1776. https://doi.org/10.1046/j.1471-4159.2000.0741773.x
Hanks LJ, Gutierrez OM, Bamman MM, Ashraf A, McCormick KL, Casazza K (2015) Circulating levels of fibroblast growth factor-21 increase with age independently of body composition indices among healthy individuals. J Clin Transl Endocrinol 2:77–82. https://doi.org/10.1016/j.jcte.2015.02.001
Hill CM, Albarado DC, Coco LG, Spann RA, Khan MS, Qualls-Creekmore E et al (2022) FGF21 is required for protein restriction to extend lifespan and improve metabolic health in male mice. Nat Commun 13:1897. https://doi.org/10.1038/s41467-022-29499-8
Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F (2010) Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab 11:206–212. https://doi.org/10.1016/j.cmet.2010.02.001
Jiang S, Yan C, Fang QC, Shao ML, Zhang YL, Liu Y et al (2014) Fibroblast growth factor 21 is regulated by the IRE1α-XBP1 branch of the unfolded protein response and counteracts endoplasmic reticulum stress-induced hepatic steatosis. J Biol Chem 289:29751–29765. https://doi.org/10.1074/jbc.M114.565960
Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ et al (2005) FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635. https://doi.org/10.1172/JCI23606
Lu X, Altshuler-Keylin S, Wang Q, Chen Y, Henrique Sponton C, Ikeda K et al (2018) Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci Signal 11:eaap8526. https://doi.org/10.1126/scisignal.aap8526
Machado SA, Pasquarelli-do-Nascimento G, da Silva DS, Farias GR, de Oliveira SI, Baptista LB et al (2022) Browning of the white adipose tissue regulation: new insights into nutritional and metabolic relevance in health and diseases. Nutr Metab (Lond) 6(19):61. https://doi.org/10.1186/s12986-022-00694-0
Markan KR (2018) Defining “FGF21 Resistance” during obesity: controversy, criteria and unresolved questions. F1000Res 7:289. https://doi.org/10.12688/f1000research.14117.1
Martínez-Sánchez N, Moreno-Navarrete JM, Contreras C, Rial-Pensado E, Fernø J, Nogueiras R et al (2017) Thyroid hormones induce browning of white fat. J Endocrinol 232:351–362. https://doi.org/10.1530/JOE-16-0425
Moore TM, Cheng L, Wolf DM, Ngo J, Segawa M, Zhu X et al (2022) Parkin regulates adiposity by coordinating mitophagy with mitochondrial biogenesis in white adipocytes. Nat Commun 3:6661. https://doi.org/10.1038/s41467-022-34468-2
Moure R, Cairó M, Morón-Ros S, Quesada-López T, Campderrós L, Cereijo R et al (2021) Levels of β-klotho determine the thermogenic responsiveness of adipose tissues: involvement of the autocrine action of FGF21. Am J Physiol Endocrinol Metab 320:E822–E834. https://doi.org/10.1152/ajpendo.00270.2020
Nedergaard J, Cannon B (2014) The browning of white adipose tissue: some burning issues. Cell Metab 20:396–407. https://doi.org/10.1016/j.cmet.2014.07.005
Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R et al (2007) BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A 104:7432–7437. https://doi.org/10.1073/pnas.0701600104
Rahman MS, Kim Y (2020) PINK1-PRKN mitophagy suppression by mangiferin promotes a brown-fat-phenotype via PKA-p38 MAPK signalling in murine C3H10T1/2 mesenchymal stem cells. Metabolism 107:154228. https://doi.org/10.1016/j.metabol.2020.154228
Salminen A, Kaarniranta K, Kauppinen A (2017) Regulation of longevity by FGF21: Interaction between energy metabolism and stress responses. Ageing Res Rev 37:79–93. https://doi.org/10.1016/j.arr.2017.05.004
Sanchis-Gomar F, Pareja-Galeano H, Santos-Lozano A, Garatachea N, Fiuza-Luces C, Venturini L et al (2015) A preliminary candidate approach identifies the combination of chemerin, fetuin-A, and fibroblast growth factors 19 and 21 as a potential biomarker panel of successful aging. Age (Dordr) 37:9776. https://doi.org/10.1007/s11357-015-9776-y
Shao W, Jin T (2022) Hepatic hormone FGF21 and its analogues in clinical trials. Chronic Dis Transl Med 8:19–25. https://doi.org/10.1016/j.cdtm.2021.08.005
Taylor D, Gottlieb RA (2017) Parkin-mediated mitophagy is downregulated in browning of white adipose tissue. Obesity (Silver Spring) 25:704–712. https://doi.org/10.1002/oby.21786
Villarroya J, Gallego-Escuredo JM, Delgado-Anglés A, Cairó M, Moure R, Gracia Mateo M et al (2018) Aging is associated with increased FGF21 levels but unaltered FGF21 responsiveness in adipose tissue. Aging Cell 17:e12822. https://doi.org/10.1111/acel.12822
Wauman J, Tavernier J (2018) The intracellular domain of the leptin receptor prevents mitochondrial depolarization and mitophagy. Biochim Biophys Acta Mol Cell Res 1865:1312–1325. https://doi.org/10.1016/j.bbamcr.2018.06.009
Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP (2007) Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 48:41–51. https://doi.org/10.1194/jlr.M600287-JLR200
Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T et al (2012) The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife 1:e00065. https://doi.org/10.7554/eLife.00065
Zoico E, Rubele S, De Caro A, Nori N, Mazzali G, Fantin F et al (2019) Brown and beige adipose tissue and aging. Front Endocrinol (Lausanne) 10:368. https://doi.org/10.3389/fendo.2019.00368
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study has been supported by the grant PID2020-114112RB-I00 Ministerio de Ciencia, Innovación y Universidades, Spain co-financed by the European Regional Development Fund.
Author information
Authors and Affiliations
Contributions
J.V., M.G. and F.V. designed the study. A.D-A. and A.B-R. obtained the samples. A.D-A, M.C. and XF.G-N. performed the analytical procedures. J.V. wrote the manuscript. All authors discussed the data and approved the final version.
The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of the University of Barcelona (approval code 259/18).
Competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
1. Parkin depletion is associated with an improved systemic metabolism in aged mice.
2. Browning of white fat is maintained in aged Parkin-KO mice.
3. FGF21 functionality plays a role in the beneficial effects shown in aged Parkin-KO mice.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary figure 1.
Low resolution image (PNG 2.10 mb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Delgado-Anglés, A., Blasco-Roset, A., Godoy-Nieto, F.J. et al. Parkin depletion prevents the age-related alterations in the FGF21 system and the decline in white adipose tissue thermogenic function in mice. J Physiol Biochem 80, 41–51 (2024). https://doi.org/10.1007/s13105-023-00977-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00977-x