Abstract
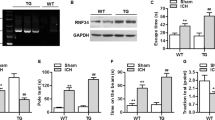
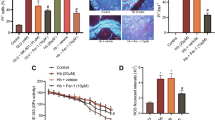
Mitochondrial dysfunction and excessive reactive oxygen species production due to impaired mitochondrial biogenesis have been proven to exacerbate secondary brain injury after intracerebral hemorrhage (ICH). The G-protein-coupled receptor 39 (GPR39) agonist TC-G 1008 has been shown to exert anti-oxidative stress effect in acute hypoxic brain injury. Herein, our study aimed to investigate the potential effects of TC-G 1008 on neuronal mitochondrial biogenesis and antioxidative stress in a mouse model of ICH and explore the underlying mechanisms. A total of 335 male C57/BL6 mice were used to establish an autologous blood-induced ICH model. Three different dosages of TC-G 1008 were administered via oral gavage at 1 h, 25 h, and 49 h post-ICH. The GPR39 siRNA and cAMP response element-binding protein (CREB) inhibitor 666–15 were administered via intracerebroventricular injection before ICH insult to explore the underlying mechanisms. Neurobehavioral function tests, Western blot, quantitative polymerase chain reaction, immunofluorescence staining, Fluoro-Jade C staining, TUNEL staining, dihydroethidium staining, transmission electron microscopy, and enzyme-linked immunosorbent assay were performed. Expression of endogenous GPR39 gradually increased in a time-dependent manner in the peri-hematoma tissues, peaking between 24 and 72 h after ICH. Treatment with TC-G 1008 significantly attenuated brain edema, hematoma size, neuronal degeneration, and neuronal death, as well as improved neurobehavioral deficits at 72 h after ICH. Moreover, TC-G 1008 upregulated the expression of mitochondrial biogenesis-related molecules, including PGC-1α, NRF1, TFAM, and mitochondrial DNA copy number, associated with antioxidative stress markers, such as Nrf2, HO-1, NQO1, SOD, CAT, and GSH-Px. Furthermore, treatment with TC-G 1008 preserved neuronal mitochondrial function and structure post-ICH. Mechanistically, the protective effects of TC-G 1008 on neuronal mitochondrial biogenesis and antioxidative stress were partially reversed by GPR39 siRNA or 666 -15. Our findings indicated that GPR39 agonist TC-G 1008 promoted mitochondrial biogenesis and improved antioxidative capability after ICH, partly through the CREB/PGC-1α signaling pathway. TC-G 1008 may be a potential therapeutic agent for patients with ICH.










Similar content being viewed by others
Data Availability
The data used in the present study are available from the corresponding author upon reasonable request.
References
Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018;392(10154):1257–68. https://doi.org/10.1096/fj.11-184531.
Chen Y, Chen S, Chang J, Wei J, Feng M, Wang R. Perihematomal edema after intracerebral hemorrhage: an update on pathogenesis, risk factors, and therapeutic advances. Front Immunol. 2021;12:740632. https://doi.org/10.3389/fimmu.2021.740632.
Li Y, Liu H, Tian C, An N, Song K, Wei Y, Sun Y, Xing Y, Gao Y. Targeting the multifaceted roles of mitochondria in intracerebral hemorrhage and therapeutic prospects. Biomed Pharmacother. 2022;148:112749. https://doi.org/10.1016/j.biopha.2022.112749.
Zheng Y, Li R, Fan X. Targeting oxidative stress in intracerebral hemorrhage: prospects of the natural products approach. Antioxidants (Basel). 2022;11(9). https://doi.org/10.3390/antiox11091811
Simmons EC, Scholpa NE, Schnellmann RG. Mitochondrial biogenesis as a therapeutic target for traumatic and neurodegenerative CNS diseases. Exp Neurol. 2020;329:113309. https://doi.org/10.1016/j.expneurol.2020.113309.
Zhang Y, Khan S, Liu Y, Wu G, Yong VW, Xue M. Oxidative stress following intracerebral hemorrhage: from molecular mechanisms to therapeutic targets. Front Immunol. 2022;13:847246. https://doi.org/10.3389/fimmu.2022.847246.
Popov L-D. Mitochondrial biogenesis: an update. J Cell Mol Med. 2020;24(9):4892–9. https://doi.org/10.1111/jcmm.15194.
Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88(2):611–38. https://doi.org/10.1152/physrev.00025.2007.
Kummer E, Ban N. Mechanisms and regulation of protein synthesis in mitochondria. Nat Rev Mol Cell Biol. 2021;22(5):307–25. https://doi.org/10.1038/s41580-021-00332-2.
Bouchez C, Devin A. Mitochondrial biogenesis and mitochondrial reactive oxygen species (ROS): a complex relationship regulated by the cAMP/PKA signaling pathway. Cells. 2019;8(4). https://doi.org/10.3390/cells8040287
Bi J, Zhang J, Ren Y, Du Z, Li Q, Wang Y, Wei S, Yang L, Zhang J, Liu C, et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019;20:296–306. https://doi.org/10.1016/j.redox.2018.10.019.
Yu H, Zhang F, Yan P, Zhang S, Lou Y, Geng Z, Li Z, Zhang Y, Xu Y, Lu Y, et al. LARP7 protects against heart failure by enhancing mitochondrial biogenesis. Circulation. 2021;143(20):2007–22. https://doi.org/10.1161/CIRCULATIONAHA.120.050812.
Chen Y, Yang Y, Liu Z, He L. Adiponectin promotes repair of renal tubular epithelial cells by regulating mitochondrial biogenesis and function. Metabolism. 2022;128:154959. https://doi.org/10.1016/j.metabol.2021.154959.
Fan H, Ding R, Liu W, Zhang X, Li R, Wei B, Su S, Jin F, Wei C, He X, et al. Heat shock protein 22 modulates NRF1/TFAM-dependent mitochondrial biogenesis and DRP1-sparked mitochondrial apoptosis through AMPK-PGC1α signaling pathway to alleviate the early brain injury of subarachnoid hemorrhage in rats. Redox Biol. 2021;40:101856. https://doi.org/10.1016/j.redox.2021.101856.
Egerod KL, Holst B, Petersen PS, Hansen JB, Mulder J, Hökfelt T, Schwartz TW. GPR39 splice variants versus antisense gene LYPD1: expression and regulation in gastrointestinal tract, endocrine pancreas, liver, and white adipose tissue. Mol Endocrinol. 2007;21(7):1685–98. https://doi.org/10.1096/fj.11-184531.
Petersen PS, Jin C, Madsen AN, Rasmussen M, Kuhre R, Egerod KL, Nielsen LB, Schwartz TW, Holst B. Deficiency of the GPR39 receptor is associated with obesity and altered adipocyte metabolism. FASEB J. 2011;25(11):3803–14. https://doi.org/10.1096/fj.11-184531.
Ishitobi Y, Akiyoshi J, Honda S, Ninomiya T, Kanehisa M, Tanaka Y, Tsuru J, Isogawa K, Kitamura H, Fujikura Y. Administration of antisense DNA for GPR39-1b causes anxiolytic-like responses and appetite loss in rats. Neurosci Res. 2012;72(3):257–62. https://doi.org/10.1016/j.neures.2011.12.002.
Sunuwar L, Medini M, Cohen L, Sekler I, Hershfinkel M. Correction to 'The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis'. Philos Trans R Soc Lond B Biol Sci. 2016;371(1703). https://doi.org/10.1098/rstb.2016.0331.
Jiang Y, Li T, Wu Y, Xu H, Xie C, Dong Y, Zhong L, Wang Z, Zhao H, Zhou Y, et al. GPR39 overexpression in OSCC Promotes YAP-sustained malignant progression. J Dent Res. 2020;99(8):949–58. https://doi.org/10.1177/0022034520915877.
Satianrapapong W, Pongkorpsakol P, Muanprasat C. A G-protein coupled receptor 39 agonist stimulates proliferation of keratinocytes via an ERK-dependent pathway. Biomed Pharmacother. 2020;127:110160. https://doi.org/10.1016/j.biopha.2020.110160.
Farbood Y, Sarkaki A, Mahdavinia M, Ghadiri A, Teimoori A, Seif F, Dehghani MA, Navabi SP. Protective effects of co-administration of zinc and selenium against streptozotocin-induced alzheimer’s disease: behavioral, mitochondrial oxidative stress, and GPR39 expression alterations in rats. Neurotox Res. 2020;38(2):398–407. https://doi.org/10.1007/s12640-020-00226-9.
Mo F, Tang Y, Du P, Shen Z, Yang J, Cai M, Zhang Y, Li H, Shen H. GPR39 protects against corticosterone-induced neuronal injury in hippocampal cells through the CREB-BDNF signaling pathway. J Affect Disord. 2020;272:474–84. https://doi.org/10.1016/j.jad.2020.03.137.
Xie S, Jiang X, Doycheva DM, Shi H, Jin P, Gao L, Liu R, Xiao J, Hu X, Tang J, et al. Activation of GPR39 with TC-G 1008 attenuates neuroinflammation via SIRT1/PGC-1α/Nrf2 pathway post-neonatal hypoxic-ischemic injury in rats. J Neuroinflammation. 2021;18(1):226. https://doi.org/10.1186/s12974-021-02289-7.
Xu Y, Zhang WH, Allen EM, Fedorov LM, Barnes AP, Qian ZY, Bah TM, Li Y, Wang RK, Shangraw RE, et al. GPR39 knockout worsens microcirculatory response to experimental stroke in a sex-dependent manner. Transl Stroke Res. 2023;14(5):766–75. https://doi.org/10.1007/s12975-022-01093-6.
Imai T, Matsubara H, Hara H. Potential therapeutic effects of Nrf2 activators on intracranial hemorrhage. J Cereb Blood Flow Metab. 2021;41(7):1483–500. https://doi.org/10.1177/0271678X20984565.
Gu L, Sun M, Li R, Tao Y, Luo X, Xu J, Wu X, Xie Z. Activation of RKIP binding ASC attenuates neuronal pyroptosis and brain injury via Caspase-1/GSDMD signaling pathway after intracerebral hemorrhage in mice. Transl Stroke Res. 2022;13(6):1037–54. https://doi.org/10.1007/s12975-022-01009-4.
Li R, Zhang X, Gu L, Yuan Y, Luo X, Shen W, Xie Z. CDGSH iron sulfur domain 2 over-expression alleviates neuronal ferroptosis and brain injury by inhibiting lipid peroxidation via AKT/mTOR pathway following intracerebral hemorrhage in mice. J Neurochem. 2023;165(3):426–44. https://doi.org/10.1111/jnc.15785.
Wu X, Fu S, Liu Y, Luo H, Li F, Wang Y, Gao M, Cheng Y, Xie Z. NDP-MSH binding melanocortin-1 receptor ameliorates neuroinflammation and BBB disruption through CREB/Nr4a1/NF-κB pathway after intracerebral hemorrhage in mice. J Neuroinflammation. 2019;16(1):192. https://doi.org/10.1186/s12974-019-1591-4.
Zeng J, Chen Y, Ding R, Feng L, Fu Z, Yang S, Deng X, Xie Z, Zheng S. Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J Neuroinflammation. 2017;14(1):119. https://doi.org/10.1186/s12974-017-0895-5.
Huang D-D, Fan S-D, Chen X-Y, Yan X-L, Zhang X-Z, Ma B-W, Yu D-Y, Xiao W-Y, Zhuang C-L, Yu Z. Nrf2 deficiency exacerbates frailty and sarcopenia by impairing skeletal muscle mitochondrial biogenesis and dynamics in an age-dependent manner. Exp Gerontol. 2019;119:61–73. https://doi.org/10.1016/j.exger.2019.01.022.
Gu L, Sun M, Li R, Zhang X, Tao Y, Yuan Y, Luo X, Xie Z. Didymin suppresses microglia pyroptosis and neuroinflammation through the Asc/Caspase-1/GSDMD pathway following experimental intracerebral hemorrhage. Front Immunol. 2022;13:810582. https://doi.org/10.3389/fimmu.2022.810582.
Suzuki H. How to promote hemoglobin scavenging or clearance and detoxification in hemorrhagic stroke. Transl Stroke Res. 2023;14(5):625–7. https://doi.org/10.1007/s12975-022-01075-8.
Xia F, Keep RF, Ye F, Holste KG, Wan S, Xi G, Hua Y. The fate of erythrocytes after cerebral hemorrhage. Transl Stroke Res. 2022;13(5):655–64. https://doi.org/10.1007/s12975-021-00980-8.
Li X, Chen G. Mitochondrial-based therapeutic strategies for intracerebral hemorrhage. Transl Stroke Res. 2022;13(2):214–5. https://doi.org/10.1007/s12975-021-00966-6.
Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80(1):315–60. https://doi.org/10.1152/physrev.2000.80.1.315.
Cheng X-T, Huang N, Sheng Z-H. Programming axonal mitochondrial maintenance and bioenergetics in neurodegeneration and regeneration. Neuron. 2022;110(12):1899–923. https://doi.org/10.1016/j.neuron.2022.03.015.
Chen W, Guo C, Feng H, Chen Y. Mitochondria: novel mechanisms and therapeutic targets for secondary brain injury after intracerebral hemorrhage. Front Aging Neurosci. 2020;12:615451. https://doi.org/10.3389/fnagi.2020.615451.
Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. https://doi.org/10.1161/CIRCRESAHA.117.311401.
Wang J, Zhou H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta Pharm Sin B. 2020;10(10):1866–79. https://doi.org/10.1016/j.apsb.2020.03.004.
Muneoka S, Goto M, Nishimura T, Enomoto K, Kadoshima-Yamaoka K, Tomimori Y. G protein-coupled receptor 39 agonist improves concanavalin A-induced hepatitis in mice. Biol Pharm Bull. 2019;42(8):1415–8. https://doi.org/10.1248/bpb.b18-00982.
Xu Y, Wang M, Xie Y, Jiang Y, Liu M, Yu S, Wang B, Liu Q. Activation of GPR39 with the agonist TC-G 1008 ameliorates ox-LDL-induced attachment of monocytes to endothelial cells. Eur J Pharmacol. 2019;858:172451. https://doi.org/10.1016/j.ejphar.2019.172451.
Xu Y, Barnes AP, Alkayed NJ. Role of GPR39 in neurovascular homeostasis and disease. Int J Mol Sci. 2021;22(15). https://doi.org/10.3390/ijms22158200
Li J, Shi X, Chen Z, Xu J, Zhao R, Liu Y, Wen Y, Chen L. Aldehyde dehydrogenase 2 alleviates mitochondrial dysfunction by promoting PGC-1α-mediated biogenesis in acute kidney injury. Cell Death Dis. 2023;14(1):45. https://doi.org/10.1038/s41419-023-05557-x.
Sharma DR, Sunkaria A, Wani WY, Sharma RK, Verma D, Priyanka K, Bal A, Gill KD. Quercetin protects against aluminium induced oxidative stress and promotes mitochondrial biogenesis via activation of the PGC-1α signaling pathway. Neurotoxicology. 2015;51:116–37. https://doi.org/10.1016/j.neuro.2015.10.002.
Vekaria HJ, Hubbard WB, Scholpa NE, Spry ML, Gooch JL, Prince SJ, Schnellmann RG, Sullivan PG. Formoterol, a β2-adrenoreceptor agonist, induces mitochondrial biogenesis and promotes cognitive recovery after traumatic brain injury. Neurobiol Dis. 2020;140:104866. https://doi.org/10.1016/j.nbd.2020.104866.
Zhang L, Tan X, Song F, Li D, Wu J, Gao S, Sun J, Liu D, Zhou Y, Mei W. Activation of G-protein-coupled receptor 39 reduces neuropathic pain in a rat model. Neural Regen Res. 2024;19(3):687–96. https://doi.org/10.4103/1673-5374.380905.
Jing W, Sun W, Zhang N, Zhao C, Yan X. The protective effects of the GPR39 agonist TC-G 1008 against TNF-α-induced inflammation in human fibroblast-like synoviocytes (FLSs). Eur J Pharmacol. 2019;865:172663. https://doi.org/10.1016/j.ejphar.2019.172663.
Shao L, Chen S, Ma L. Secondary brain injury by oxidative stress after cerebral hemorrhage: recent advances. Front Cell Neurosci. 2022;16:853589. https://doi.org/10.3389/fncel.2022.853589.
Chen S, Li L, Peng C, Bian C, Ocak PE, Zhang JH, Yang Y, Zhou D, Chen G, Luo Y. Targeting oxidative stress and inflammatory response for blood-brain barrier protection in intracerebral hemorrhage. Antioxid Redox Signal. 2022;37(1–3):115–34. https://doi.org/10.1089/ars.2021.0072.
Yang P, Feng Q, Meng L, Tang R, Jiang Y, Liu H, Si H, Li M. The mechanism underlying the TC-G 1008 rescue of reactive oxygen species (ROS)-induced osteoblast apoptosis by the upregulation of peroxiredoxin 1. Int J Biochem Cell Biol. 2022;151:106276. https://doi.org/10.1016/j.biocel.2022.106276.
Wang D, Wang Y, Zou X, Shi Y, Liu Q, Huyan T, Su J, Wang Q, Zhang F, Li X, et al. FOXO1 inhibition prevents renal ischemia-reperfusion injury via cAMP-response element binding protein/PPAR-γ coactivator-1α-mediated mitochondrial biogenesis. Br J Pharmacol. 2020;177(2):432–48. https://doi.org/10.1111/bph.14878.
Lee J, Kim C-H, Simon DK, Aminova LR, Andreyev AY, Kushnareva YE, Murphy AN, Lonze BE, Kim K-S, Ginty DD, et al. Mitochondrial cyclic AMP response element-binding protein (CREB) mediates mitochondrial gene expression and neuronal survival. J Biol Chem. 2005;280(49):40398–401. https://doi.org/10.1074/jbc.C500140200.
Zhao L-J, Zhang S-F. Activation of TGR5 promotes mitochondrial biogenesis in human aortic endothelial cells. Biochem Biophys Res Commun. 2018;500(4):952–7. https://doi.org/10.1016/j.bbrc.2018.04.210.
Yan J, Xu W, Lenahan C, Huang L, Wen J, Li G, Hu X, Zheng W, Zhang JH, Tang J. CCR5 Activation Promotes NLRP1-Dependent Neuronal Pyroptosis via CCR5/PKA/CREB Pathway After Intracerebral Hemorrhage. Stroke. 2021;52(12):4021–32. https://doi.org/10.1161/STROKEAHA.120.033285.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82201470), Chongqing Natural Science Foundation Project (No. CSTB2023NSCQ-MSX0107 and No. cstc2020jcyj-msxmX0225), and Chongqing Postdoctoral Science Foundation (No.2022CQBSHTB3097).
Author information
Authors and Affiliations
Contributions
The research was conceived and designed by Zhongyi Zhang and Lingui Gu. Zhongyi Zhang, Xingyu Zhang, and Jinyu Dai conducted the research. Ye Yuan and Yuguang Tang analyzed the data. Zhongyi Zhang, Yutong Zhao, Yihao Tao, and Zongyi Xie drafted and revised the manuscript. All authors have reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This research study strictly followed the guidelines established by the United States National Institutes of Health for the care and utilization of laboratory animals. Prior to commencing any testing or experimental procedures, comprehensive evaluations were conducted on the study protocols and obtained approval from Chongqing Medical University's Animal Ethics Committee (Permit number: IACUG-CQMU-2022–0015).
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Yuan, Y., Zhang, X. et al. GPR39 Agonist TC-G 1008 Promoted Mitochondrial Biogenesis and Improved Antioxidative Capability via CREB/PGC-1α Pathway Following Intracerebral Hemorrhage in Mice. Transl. Stroke Res. (2024). https://doi.org/10.1007/s12975-024-01240-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12975-024-01240-1