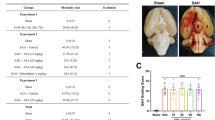
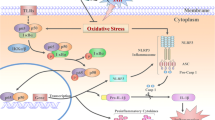
Abstract
The inflammatory response following subarachnoid hemorrhage (SAH) may lead to Early Brain Injury and subsequently contribute to poor prognosis such as cognitive impairment in patients. Currently, there is a lack of effective strategies for SAH to ameliorate inflammation and improve cognitive impairment in clinical. This study aims to examine the inhibitory impact of remote ischemic post-conditioning (RIPostC) on the body’s inflammatory response by regulating Th17/Treg cell homeostasis after SAH. The ultimate goal is to search for potential early treatment targets for SAH. The rat SAH models were made by intravascular puncture of the internal carotid artery. The intervention of RIPostC was administered for three consecutive days immediately after successful modeling. Behavioral experiments including the Morris water maze and Y-maze tests were conducted to assess cognitive functions such as spatial memory, working memory, and learning abilities 2 weeks after successful modeling. The ratio of Th17 cells and Treg cells in the blood was detected using flow cytometry. Immunofluorescence was used to observe the infiltration of neutrophils into the brain. Signal transducers and activators of transcription 5 (STAT5) and signal transducers and activators of transcription 3 (STAT3) phosphorylation levels, receptor-related orphan receptor gamma-t (RORγt), and forkhead box protein P3 (Foxp3) levels were detected by Western blot. The levels of anti-inflammatory factors (IL-2, IL-10, IL-5, etc.) and pro-inflammatory factors (IL-6, IL-17, IL-18, TNF-α, IL-14, etc.) in blood were detected using Luminex Liquid Suspension Chip Assay. RIPostC significantly improved the cognitive impairment caused by SAH in rats. The results showed that infiltration of Th17 cells and neutrophils into brain tissue increased after SAH, leading to the release of pro-inflammatory factors (IL-6, IL-17, IL-18, and TNF-α). This response can be inhibited by RIPostC. Additionally, RIPostC facilitates the transfer of Treg from blood to the brain and triggers the release of anti-inflammatory (IL-2, IL-10, and IL-5) factors to suppress the inflammation following SAH. Finally, it was found that RIPostC increased the phosphorylation of STAT5 while decreasing the phosphorylation of STAT3. RIPostC reduces inflammation after SAH by partially balancing Th17/Treg cell homeostasis, which may be related to downregulation of STAT3 and upregulation of STAT5 phosphorylation, which ultimately alleviates cognitive impairment in rats. Targeting Th17/Treg cell homeostasis may be a promising strategy for early SAH treatment.






Similar content being viewed by others
Data Availability
Data are available upon reasonable request.
Abbreviations
- SAH:
-
Subarachnoid hemorrhage
- RIPostC:
-
Remote ischemic post-conditioning
- STAT:
-
Signal transducers and activators of transcription
- STAT5:
-
Signal transducers and activators of transcription 5
- STAT3:
-
Signal transducers and activators of transcription 3
- RORγt:
-
Receptor-related orphan receptor gamma-t
- Foxp3:
-
Forkhead box protein P3
- IL-2:
-
Interleukin-2
- IL-4:
-
Interleukin-4
- IL-5:
-
Interleukin-5
- IL-6:
-
Interleukin-6
- IL-10:
-
Interleukin-10
- IL-17:
-
Interleukin-17
- IL-18:
-
Interleukin-18
- TNF-α:
-
Tumor necrosis factor alpha
- VEGF:
-
Vascular endothelial growth factor
- JAK:
-
Janus kinase
References
Mangonic AA, Zinellu A. “A systematic review and meta-analysis of serum concentrations of ischaemia-modified albumin in acute ischaemic stroke, intracerebral haemorrhage, and subarachnoid haemorrhage”. Biomolecules. 2022;12(5). https://doi.org/10.3390/biom12050653
Claassen J, Park S. Spontaneous subarachnoid haemorrhage. Lancet. 2022;400(10355):846–62. https://doi.org/10.1016/s0140-6736(22)00938-2.
Gerner ST, Reichl J, Custal C, et al. Long-term complications and influence on outcome in patients surviving spontaneous subarachnoid hemorrhage. Cerebrovasc Dis. 2020;49(3):307–15. https://doi.org/10.1159/000508577.
Gris T, Laplante P, Thebault P, et al. Innate immunity activation in the early brain injury period following subarachnoid hemorrhage. J Neuroinflammation. 2019;16(1):253. https://doi.org/10.1186/s12974-019-1629-7.
Chen J, Zheng ZV, Lu G, et al. Microglia activation, classification and microglia-mediated neuroinflammatory modulators in subarachnoid hemorrhage. Neural Regen Res. 2022;17(7):1404–11. https://doi.org/10.4103/1673-5374.330589.
Chen J, Wong GKC. Microglia accumulation and activation after subarachnoid hemorrhage. Neural Regen Res. 2021;16(8):1531–2. https://doi.org/10.4103/1673-5374.303028.
Tian Y, Liu B, Li Y, et al. Activation of RARα receptor attenuates neuroinflammation after SAH via promoting M1-to-M2 phenotypic polarization of microglia and regulating Mafb/Msr1/PI3K-Akt/NF-κB pathway. Front Immunol. 2022;13:839796. https://doi.org/10.3389/fimmu.2022.839796.
Yang LY, Chen YR, Lee JE, et al. Dental pulp stem cell-derived conditioned medium alleviates subarachnoid hemorrhage-induced microcirculation impairment by promoting M2 microglia polarization and reducing astrocyte swelling. Transl Stroke Res. 2023;14(5):688–703. https://doi.org/10.1007/s12975-022-01083-8.
Jin J, Duan J, Du L, et al. Inflammation and immune cell abnormalities in intracranial aneurysm subarachnoid hemorrhage (SAH): relevant signaling pathways and therapeutic strategies. Front Immunol. 2022;13:1027756. https://doi.org/10.3389/fimmu.2022.1027756.
Zeng H, Fu X, Cai J, et al. Neutrophil extracellular traps may be a potential target for treating early brain injury in subarachnoid hemorrhage. Transl Stroke Res. 2022;13(1):112–31. https://doi.org/10.1007/s12975-021-00909-1.
Moraes L, Trias N, Brugnini A, et al. TH17/Treg imbalance and IL-17A increase after severe aneurysmal subarachnoid hemorrhage. J Neuroimmunol. 2020;346:577310. https://doi.org/10.1016/j.jneuroim.2020.577310.
Chaudhry SR, Kahlert UD, Kinfe TM, et al. Differential polarization and activation dynamics of systemic T helper cell subsets after aneurysmal subarachnoid hemorrhage (SAH) and during post-SAH complications. Sci Rep. 2021;11(1):14226. https://doi.org/10.1038/s41598-021-92873-x.
Hao X, Zeng Z, Liang L, et al. The role of neutrophil extracellular traps in early microthrombosis and brain injury after subarachnoid hemorrhage in mice. Transl Stroke Res. 2023;14(5):752–65. https://doi.org/10.1007/s12975-022-01074-9.
Chen HS, Cui Y, Li XQ, et al. Effect of remote ischemic conditioning vs usual care on neurologic function in patients with acute moderate ischemic stroke: the RICAMIS randomized clinical trial. Jama. 2022;328(7):627–36. https://doi.org/10.1001/jama.2022.13123.
Nikkola E, Laiwalla A, Ko A, et al. Remote ischemic conditioning alters methylation and expression of cell cycle genes in aneurysmal subarachnoid hemorrhage. Stroke. 2015;46(9):2445–51. https://doi.org/10.1161/strokeaha.115.009618.
Zhao W, Jiang F, Li S, et al. Safety and efficacy of remote ischemic conditioning for the treatment of intracerebral hemorrhage: a proof-of-concept randomized controlled trial. Int J Stroke. 2022;17(4):425–33. https://doi.org/10.1177/17474930211006580.
Saito M, Hoshino T, Ishizuka K, et al. Remote ischemic conditioning enhances collateral circulation through leptomeningeal anastomosis and diminishes early ischemic lesions and infarct volume in middle cerebral artery occlusion. Transl Stroke Res. 2022. https://doi.org/10.1007/s12975-022-01108-2.
Mohammad Seyedsaadat S, Kallmes DF, Brinjikji W. Remote ischemic conditioning approach for the treatment of ischemic stroke. Neural Regen Res. 2020;15(6):1033–4. https://doi.org/10.4103/1673-5374.270303.
Ripley AJ, Jeffers MS, McDonald MW, et al. Neuroprotection by remote ischemic conditioning in rodent models of focal ischemia: a systematic review and meta-analysis. Transl Stroke Res. 2021;12(3):461–73. https://doi.org/10.1007/s12975-020-00882-1.
Sun YY, Zhu HJ, Zhao RY, et al. Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice. Redox Biol. 2023;66:102852. https://doi.org/10.1016/j.redox.2023.102852.
Hu X, Lv T, Yang SF, et al. Limb remote ischemic post-conditioning reduces injury and improves long-term behavioral recovery in rats following subarachnoid hemorrhage: possible involvement of the autophagic process. Mol Med Rep. 2018;17(1):21–30. https://doi.org/10.3892/mmr.2017.7858.
Nadeem M, Kindelin A, Mahady L, et al. Remote ischemic post-conditioning therapy is protective in mouse model of traumatic optic neuropathy. Neuromolecular Med. 2021;23(3):371–82. https://doi.org/10.1007/s12017-020-08631-1.
Liu C, Yang J, Zhang C, et al. Remote ischemic conditioning reduced cerebral ischemic injury by modulating inflammatory responses and ERK activity in type 2 diabetic mice. Neurochem Int. 2020;135:104690. https://doi.org/10.1016/j.neuint.2020.104690.
Zhao P, Li J, Tian Y, et al. Restoring Th17/Treg balance via modulation of STAT3 and STAT5 activation contributes to the amelioration of chronic obstructive pulmonary disease by Bufei Yishen formula. J Ethnopharmacol. 2018;217:152–62. https://doi.org/10.1016/j.jep.2018.02.023.
Wang F, Yang Y, Li Z, et al. Mannan-binding lectin regulates the Th17/Treg axis through JAK/STAT and TGF-β/SMAD signaling against Candida albicans infection. J Inflamm Res. 2022;15:1797–810. https://doi.org/10.2147/jir.S344489.
Kumar R, Theiss AL, Venuprasad K. RORγt protein modifications and IL-17-mediated inflammation. Trends Immunol. 2021;42(11):1037–50. https://doi.org/10.1016/j.it.2021.09.005.
Sheng W, Yang F, Zhou Y, et al. STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res. 2014;24(12):1387–402. https://doi.org/10.1038/cr.2014.154.
Xu J, Li Q, Xu CY, et al. Obstructive sleep apnea aggravates neuroinflammation and pyroptosis in early brain injury following subarachnoid hemorrhage via ASC/HIF-1α pathway. Neural Regen Res. 2022;17(11):2537–43. https://doi.org/10.4103/1673-5374.339000.
Alpdogan S, Sander T, Zhang R, et al. Meta-review on perforation model of subarachnoid hemorrhage in mice: filament material as a possible moderator of mortality. Transl Stroke Res. 2022. https://doi.org/10.1007/s12975-022-01106-4.
Ren C, Li S, Wang B, et al. Limb remote ischemic conditioning increases Notch signaling activity and promotes arteriogenesis in the ischemic rat brain. Behav Brain Res. 2018;340:87–93. https://doi.org/10.1016/j.bbr.2016.10.036.
Othman MZ, Hassan Z. Che Has AT “Morris water maze: a versatile and pertinent tool for assessing spatial learning and memory.” Exp Anim. 2022;71(3):264–80. https://doi.org/10.1538/expanim.21-0120.
Cao Y, Wang Y, Li X, et al. MCC950 ameliorates cognitive function by reducing white matter microstructure damage in rats after SAH. Brain Res Bull. 2023;202:110743. https://doi.org/10.1016/j.brainresbull.2023.110743.
Ke DQ, Chen ZY, Li ZL, et al. Target inhibition of caspase-8 alleviates brain damage after subarachnoid hemorrhage. Neural Regen Res. 2020;15(7):1283–9. https://doi.org/10.4103/1673-5374.272613.
Wang Y, Yang X, Cao Y, et al. Electroacupuncture promotes remyelination and alleviates cognitive deficit via promoting OPC differentiation in a rat model of subarachnoid hemorrhage. Metab Brain Dis. 2023;38(2):687–98. https://doi.org/10.1007/s11011-022-01102-5.
Liang Z, Qiu L, Wang X, et al. Effects of remote ischemic postconditioning on the pro-inflammatory neutrophils of peripheral blood in acute cerebral infarction. Aging (Albany NY). 2023;15(10):4481–97. https://doi.org/10.18632/aging.204751.
Liu C, Yang J, Zhang C, et al. The changes of systemic immune responses during the neuroprotection induced by remote ischemic postconditioning against focal cerebral ischemia in mice. Neurol Res. 2019;41(1):26–36. https://doi.org/10.1080/01616412.2018.1523037.
Ramagiri S, Taliyan R. Remote limb ischemic post conditioning during early reperfusion alleviates cerebral ischemic reperfusion injury via GSK-3β/CREB/ BDNF pathway. Eur J Pharmacol. 2017;803:84–93. https://doi.org/10.1016/j.ejphar.2017.03.028.
Tack RWP, Amboni C, van Nuijs D, et al. Inflammation, anti-inflammatory interventions, and post-stroke cognitive impairment: a systematic review and meta-analysis of human and animal studies. Transl Stroke Res. 2023. https://doi.org/10.1007/s12975-023-01218-5.
Zhu H, Hu S, Li Y, et al. Interleukins and ischemic stroke. Front Immunol. 2022;13:828447. https://doi.org/10.3389/fimmu.2022.828447.
Zhang H, Xia Y, Ye Q, et al. In vivo expansion of regulatory T cells with IL-2/IL-2 antibody complex protects against transient ischemic stroke. J Neurosci. 2018;38(47):10168–79. https://doi.org/10.1523/jneurosci.3411-17.2018.
Xu JY, Xiong YY, Tang RJ, et al. Interleukin-5-induced eosinophil population improves cardiac function after myocardial infarction. Cardiovasc Res. 2022;118(9):2165–78. https://doi.org/10.1093/cvr/cvab237.
Allgire E, Ahlbrand RA, Nawreen N, et al. Altered fear behavior in aeroallergen house dust mite exposed C57Bl/6 mice: a model of Th2-skewed airway inflammation. Neuroscience. 2023;528:75–88. https://doi.org/10.1016/j.neuroscience.2023.07.022.
Zhu X, Zhu J. “CD4 T helper cell subsets and related human immunological disorders”. Int J Mol Sci. 2020;21(21). https://doi.org/10.3390/ijms21218011
Doeppner TR, Zechmeister B, Kaltwasser B, et al. Very delayed remote ischemic post-conditioning induces sustained neurological recovery by mechanisms involving enhanced angioneurogenesis and peripheral immunosuppression reversal. Front Cell Neurosci. 2018;12:383. https://doi.org/10.3389/fncel.2018.00383.
Leung CH, Rizoli SB, Trypcic S, et al. Effect of remote ischemic conditioning on the immune-inflammatory profile in patients with traumatic hemorrhagic shock in a randomized controlled trial. Sci Rep. 2023;13(1):7025. https://doi.org/10.1038/s41598-023-33681-3.
Wang L, Ren C, Li Y, et al. Remote ischemic conditioning enhances oxygen supply to ischemic brain tissue in a mouse model of stroke: role of elevated 2,3-biphosphoglycerate in erythrocytes. J Cereb Blood Flow Metab. 2021;41(6):1277–90. https://doi.org/10.1177/0271678x20952264.
Mills KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. 2023;23(1):38–54. https://doi.org/10.1038/s41577-022-00746-9.
Zhao Z, Wang Y, Gao Y, et al. The PRAK-NRF2 axis promotes the differentiation of Th17 cells by mediating the redox homeostasis and glycolysis. Proc Natl Acad Sci U S A. 2023;120(19):e2212613120. https://doi.org/10.1073/pnas.2212613120.
Minns D, Smith KJ, Alessandrini V, et al. The neutrophil antimicrobial peptide cathelicidin promotes Th17 differentiation. Nat Commun. 2021;12(1):1285. https://doi.org/10.1038/s41467-021-21533-5.
Yu HH, Ma XT, Ma X, et al. Remote limb ischemic postconditioning protects against ischemic stroke by promoting regulatory T cells thriving. J Am Heart Assoc. 2021;10(22):e023077. https://doi.org/10.1161/jaha.121.023077.
Qian ZY, Kong RY, Zhang S, et al. Ruxolitinib attenuates secondary injury after traumatic spinal cord injury. Neural Regen Res. 2022;17(9):2029–35. https://doi.org/10.4103/1673-5374.335165.
Xu AH, Yang Y, Shao Y, et al. Poly(ADP-ribose) polymerase family member 14 promotes functional recovery after spinal cord injury through regulating microglia M1/M2 polarization via STAT1/6 pathway. Neural Regen Res. 2023;18(8):1809–17. https://doi.org/10.4103/1673-5374.357909.
Alhadidi Q, Shah ZA. Cofilin mediates LPS-induced microglial cell activation and associated neurotoxicity through activation of NF-κB and JAK-STAT pathway. Mol Neurobiol. 2018;55(2):1676–91. https://doi.org/10.1007/s12035-017-0432-7.
Feng X, Li M, Lin Z, et al. Tetramethylpyrazine promotes axonal remodeling and modulates microglial polarization via JAK2-STAT1/3 and GSK3-NFκB pathways in ischemic stroke. Neurochem Int. 2023;170:105607. https://doi.org/10.1016/j.neuint.2023.105607.
Dong Z, Cao L, Guo L, et al. [Retracted] CCL26 regulates the proportion of CD4+CD25+FOXP3+ Tregs and the production of inflammatory factors in peripheral blood mononuclear cells following acute ischemic stroke via the STAT5 pathway. Exp Ther Med. 2023;25(4):174. https://doi.org/10.3892/etm.2023.11873.
Feng L, Zhang X, Li W, et al. Proteomics reveals that Di Dang decoction can regulate the Jak2/Stat5 signaling pathway and inhibit apoptosis by reducing the oxidative stress response in rats with acute intracerebral hemorrhagic stroke. J Ethnopharmacol. 2023;301:115816. https://doi.org/10.1016/j.jep.2022.115816.
Liu C, Arnold R, Henriques G, et al. “Inhibition of JAK-STAT signaling with baricitinib reduces inflammation and improves cellular homeostasis in progeria cells”. Cells. 2019;8(10). https://doi.org/10.3390/cells8101276
Jones DM, Read KA, Oestreich KJ. Dynamic roles for IL-2-STAT5 signaling in effector and regulatory CD4(+) T cell populations. J Immunol. 2020;205(7):1721–30. https://doi.org/10.4049/jimmunol.2000612.
Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. https://doi.org/10.1016/j.immuni.2009.03.019.
Acknowledgements
Firstly, we would like to thank the Chongqing Key Laboratory for Major Neuropsychiatric Disorders for providing a platform and technical support for the research. Secondly, we would like to express our gratitude to the Experimental Animal Center of Chongqing Medical University for providing us with experimental animals. Finally, we would like to appreciate Dr. Xiaochuan Sun, Director of the Department of Theology and Gynecology of the First Affiliated Hospital of Chongqing Medical University, Dr. Jiru Zhou, Dr. Linna Gu, Dr. Lin Wang, Dr. Zhao Li, Master Yunchuan Cao, and Master Bo Zeng for providing experimental and technical guidance for this study.
Funding
This work was supported partly by grants from the National Natural Science Foundation of China (Grant number 82071332), the Key Project of Chongqing Science and Health Joint Medical Research Project (2020GDRC024), and the Chongqing Natural Science Foundation Joint Fund for Innovation and Development (CSTB2023NSCQ-LZX0041).
Author information
Authors and Affiliations
Contributions
In this article, Yajun Zhu participated in research design, experimental arrangement, data statistics and analysis, and manuscript preparation. Xiaoguo Li participated in the Luminex Liquid Suspension Chip Assay. DaoChen Wen participated in Western blotting. Zichao Haung participated in flow cytometry. Jin Yan, Zhaosi Zhang and Yingwen Wang participated in the construction of the rat SAH model. Zongduo Guo participated in manuscript editing and manuscript review.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Y., Li, X., Wen, D. et al. Remote Ischemic Post-conditioning Reduces Cognitive Impairment in Rats Following Subarachnoid Hemorrhage: Possible Involvement in STAT3/STAT5 Phosphorylation and Th17/Treg Cell Homeostasis. Transl. Stroke Res. (2024). https://doi.org/10.1007/s12975-024-01235-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12975-024-01235-y