Abstract
Ischemic stroke is the leading cause of morbidity and mortality with a significant health burden worldwide and few treatment options. Among the short- and long-term effects of ischemic stroke is the cardiovascular sympathetic autonomic dysfunction, presented in part as the by-product of the ischemic damage to the noradrenergic centers of the brain. Unlike high levels in the plasma, the brain may face suboptimal levels of norepinephrine (NE), with adverse effects on the clinical and functional outcomes of ischemic stroke. The intravenous administration of NE and other sympathomimetic agents, in an attempt to increase cerebral perfusion pressure, often aggravates the ischemia-induced rise in blood pressure (BP) with life-threatening consequences for stroke patients, the majority of whom present with hypertension at the time of admission. Unlike the systemic administration, the central administration of NE reduces BP while exerting anti-inflammatory and neuroprotective effects. These characteristics of centrally administered NE, combined with the short latency of response, make it an ideal candidate for use in the acute phase of stroke, followed by the use of centrally acting noradrenergic agonists, such as NE reuptake inhibitors and B2-adrenergic receptor agonists for stroke rehabilitation. In addition, a number of nonpharmacological strategies, such as transcutaneous vagus nerve stimulation (tVNS) and trigeminal nerve stimulation (TNS), have the potential to enhance the central noradrenergic functional activities and improve stroke clinical outcomes. Many factors could influence the efficacy of the noradrenergic treatment in stroke patients. These factors include the type of the noradrenergic agent; the dose, frequency, and duration of administration; the timing of administration in relation to the acute event; and the site and characteristics of the ischemic lesions. Having this knowledge, combined with the better understanding of the regulation of noradrenergic receptors in different parts of the brain, would pave the path for the successful use of the centrally acting noradrenergic agents in the management of ischemic stroke.

Similar content being viewed by others
References
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410.
Hickey JV. The clinical practice of neurological and neurosurgical nursing. 5th ed. Philadelphia: Lippincott, Williams & Wilkins; 2003.
Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–35.
Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. Prognosis of untreated strokes due to anterior circulation proximal intracranial arterial occlusions detected by use of computed tomography angiography. JAMA Neurol. 2014;71:151–7.
Smith WS, Lev MH, English JD, Camargo EC, Chou M, Johnston SC, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40:3834–40.
Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–5.
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–30.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–306.
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–95.
Deb P, Sharma S, Hassan KM. Pathophysiologic mechanisms of acute ischemic stroke: an overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17:197–218.
Turley KR, Toledo-Pereyra LH, Kothari RU. Molecular mechanisms in the pathogenesis and treatment of acute ischemic stroke. J Invest Surg. 2005;18:207–18.
Yu G, Wu F, Wang ES. BQ-869, a novel NMDA receptor antagonist, protects against excitotoxicity and attenuates cerebral ischemic injury in stroke. Int J Clin Exp Pathol. 2015;8:1213–25.
Douglas HA, Callaway JK, Sword J, Kirov SA, Andrew RD. Potent inhibition of anoxic depolarization by the sodium channel blocker dibucaine. J Neurophysiol. 2011;105:1482–94.
Maniskas ME, Roberts JM, Aron I, Fraser JF, Bix GJ. Stroke neuroprotection revisited: intra-arterial verapamil is profoundly neuroprotective in experimental acute ischemic stroke. J Cereb Blood Flow Metab. 2016;36:721–30.
Tuo YH, Liu Z, Chen JW, Wang QY, Li SL, Li MC, et al. NADPH oxidase inhibitor improves outcome of mechanical reperfusion by suppressing hemorrhagic transformation. J Neurointerv Surg. 2017;9:492–8.
Li X, Su L, Zhang X, Zhang C, Wang L, Li Y, et al. Ulinastatin downregulates TLR4 and NF-kB expression and protects mouse brains against ischemia/reperfusion injury. Neurol Res. 2017;39:367–73.
Korpelainen JT, Sotaniemi KA, Myllyla VV. Autonomic nervous system disorders in stroke. Clin Auton Res. 1999;9:325–33.
Castro P, Serrador JM, Rocha I, Sorond F, Azevedo E. Efficacy of cerebral autoregulation in early ischemic stroke predicts smaller infarcts and better outcome. Front Neurol. 2017;8:113.
Talman WT. Cardiovascular regulation and lesions of the central nervous system. Ann Neurol. 1985;18:1–13.
Xiong L, Tian G, Leung H, Soo YOY, Chen X, Ip VHL, et al. Autonomic dysfunction predicts clinical outcomes after acute ischemic stroke: a prospective observational study. Stroke. 2018;49:215–8.
Graff B, Gasecki D, Rojek A, Boutouyrie P, Nyka W, Laurent S, et al. Heart rate variability and functional outcome in ischemic stroke: a multiparameter approach. J Hypertens. 2013;31:1629–36.
Korpelainen JT, Sotaniemi KA, Huikuri HV, Myllya VV. Abnormal heart rate variability as a manifestation of autonomic dysfunction in hemispheric brain infarction. Stroke. 1996;27:2059–63.
Xu YH, Wang XD, Yang JJ, Zhou L, Pan YC. Changes of deceleration and acceleration capacity of heart rate in patients with acute hemispheric ischemic stroke. Clin Interv Aging. 2016;11:293–8.
Weis M, Claus D, Rechlin T, Neundorfer B. Disorders of autonomic heart rate regulation in patients with brain stem lesions. Nervenarzt. 1994;65:381–9.
Constantinescu V, Matei D, Costache V, Cuciureanu D, Arsenescu-Georgescu C. Linear and nonlinear parameters of heart rate variability in ischemic stroke patients. Neurol Neurochir Pol. 2018;52:194–206.
Beer NR, Soroker N, Bornstein NM, Leurer MK. Association between cardiac autonomic control and cognitive performance among patients post stroke and age-matched healthy controls—an exploratory pilot study. Neurol Sci. 2017;38:2037–43.
Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol. 2008;6:235–53.
Hertz L, Lovatt D, Goldman SA, Nedergaard M. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem Int. 2010;57:411–20.
Gannon M, Che P, Chen Y, Jiao K, Roberson ED, Wang Q. Noradrenergic dysfunction in Alzheimer's disease. Front Neurosci. 2015;9:220.
Polak PE, Kalinin S, Feinstein DL. Locus coeruleus damage and noradrenaline reductions in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 2011;134:665–77.
Li D, Du CY, Tang XJ, Jin YX, Lei T, Yao Y, et al. Changes of heart rate variability and impairment of learning and memory induced by cerebral ischemia/reperfusion in rats. Sheng Li Xue Bao. 2007;59:35–41.
Robinson RG, Shoemaker WJ, Schlumpf M. Time course of changes in catecholamines following right hemispheric cerebral infarction in the rat. Brain Res. 1980;181:202–8.
Iijima S, Hara K, Suga H, Nakamura S, Kameyama M. Effect of ischemia on hydroxylase cofactor (tetrahydrobiopterin) and monoamine neurotransmitters in rat brain. Stroke. 1986;17:529–33.
Weinberger J, Cohen G, Nieves-Rosa J. Nerve terminal damage in cerebral ischemia: greater susceptibility of catecholamine nerve terminals relative to serotonin nerve terminals. Stroke. 1983;14:986–9.
Fritschy JM, Grzanna R. Experimentally-induced neuron loss in the locus coeruleus of adult rats. Exp Neurol. 1991;111:123–7.
Fritschy JM, Grzanna R. Selective effects of DSP-4 on locus coeruleus axons: are there pharmacologically different types of noradrenergic axons in the central nervous system? Prog Brain Res. 1991;88:257–68.
Szot P, Miguelez C, White SS, Franklin A, Sikkema C, Wilkinson CW, et al. A comprehensive analysis of the effect of DSP4 on the locus coeruleus noradrenergic system in the rat. Neuroscience. 2010;166:279–91.
Prieto M, Giralt MT. Effects of N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP4) on alpha2-adrenoceptors which regulate the synthesis and release of noradrenaline in the rat brain. Pharmacol Toxicol. 2001;88:152–8.
Brouns R, Van Hemelrijck A, Drinkenburg WH, Van Dam D, De Surgeloose D, De Deyn PP. Excitatory amino acids and monoaminergic neurotransmitters in cerebrospinal fluid of acute ischemic stroke patients. Neurochem Int. 2010;56:865–70.
Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–19.
Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension. 2004;43:18–24.
O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–23.
Maier B, Gory B, Taylor G, Labreuche J, Blanc R, Obadia M, et al. Mortality and disability according to baseline blood pressure in acute ischemic stroke patients treated by thrombectomy: a collaborative pooled analysis. J Am Heart Assoc. 2017;6.
Morfis L, Schwartz RS, Poulos R, Howes LG. Blood pressure changes in acute cerebral infarction and hemorrhage. Stroke. 1997;28:1401–5.
P. Bonardo, F. Pantiu, A. Chertcoff, L. Leon Cejas, S. Pacha, C. Uribe Roca, et al., Blood pressure evolution in young patients with acute ischemic stroke: a new model for understanding the natural course of spontaneous hypertension? Int J Neurosci. 2017;1–6.
Dicker D, Maya I, Vasilevsky V, Gofman M, Markowitz D, Beilin V, et al. Blood pressure variability in acute ischemic stroke depends on hemispheric stroke location. Blood Press. 2006;15:151–6.
Kwarciany M, Gasecki D, Kowalczyk K, Rojek A, Laurent S, Boutouyrie P, et al. Acute hypertensive response in ischemic stroke is associated with increased aortic stiffness. Atherosclerosis. 2016;251:1–5.
Akil E, Tamam Y, Akil MA, Kaplan I, Bilik MZ, Acar A, et al. Identifying autonomic nervous system dysfunction in acute cerebrovascular attack by assessments of heart rate variability and catecholamine levels. J Neurosci Rural Pract. 2015;6:145–50.
ter Laan M, van Dijk JM, Elting JW, Staal MJ, Absalom AR. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth. 2013;111:361–7.
Strittmatter M, Meyer S, Fischer C, Georg T, Schmitz B. Location-dependent patterns in cardio-autonomic dysfunction in ischaemic stroke. Eur Neurol. 2003;50:30–8.
Oto J, Suzue A, Inui D, Fukuta Y, Hosotsubo K, Torii M, et al. Plasma proinflammatory and anti-inflammatory cytokine and catecholamine concentrations as predictors of neurological outcome in acute stroke patients. J Anesth. 2008;22:207–12.
Schulze J, Vogelgesang A, Dressel A. Catecholamines, steroids and immune alterations in ischemic stroke and other acute diseases. Aging Dis. 2014;5:327–39.
Segura-Chama P, Lopez-Bistrain P, Perez-Armendariz EM, Jimenez-Perez N, Millan-Aldaco D, Hernandez-Cruz A. Enhanced Ca(2+)-induced Ca(2+) release from intracellular stores contributes to catecholamine hypersecretion in adrenal chromaffin cells from spontaneously hypertensive rats. Pflugers Arch. 2015;467:2307–23.
Fhaner MJ, Galligan JJ, Swain GM. Increased catecholamine secretion from single adrenal chromaffin cells in DOCA-salt hypertension is associated with potassium channel dysfunction. ACS Chem Neurosci. 2013;4:1404–13.
Grobecker H, Saavedra JM, Dominiak P. Catecholamines in experimental and essential hypertension. Heart Hypertens. 1981;109–21.
Musso NR, Brenci S, Setti M, Indiveri F, Lotti G. Catecholamine content and in vitro catecholamine synthesis in peripheral human lymphocytes. J Clin Endocrinol Metab. 1996;81:3553–7.
Qiu YH, Cheng C, Dai L, Peng YP. Effect of endogenous catecholamines in lymphocytes on lymphocyte function. J Neuroimmunol. 2005;167:45–52.
Cosentino M, Marino F, Bombelli R, Ferrari M, Rasini E, Lecchini S, et al. Stimulation with phytohaemagglutinin induces the synthesis of catecholamines in human peripheral blood mononuclear cells: role of protein kinase C and contribution of intracellular calcium. J Neuroimmunol. 2002;125:125–33.
Kohm AP, Tang Y, Sanders VM, Jones SB. Activation of antigen-specific CD4+ Th2 cells and B cells in vivo increases norepinephrine release in the spleen and bone marrow. J Immunol. 2000;165:725–33.
Kopin IJ, Breese GR, Krauss KR, Weise VK. Selective release of newly synthesized norepinephrine from the cat spleen during sympathetic nerve stimulation. J Pharmacol Exp Ther. 1968;161:271–8.
Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004;45:38–78.
Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174.
Mori K, Ozaki E, Zhang B, Yang L, Yokoyama A, Takeda I, et al. Effects of norepinephrine on rat cultured microglial cells that express alpha1, alpha2, beta1 and beta2 adrenergic receptors. Neuropharmacology. 2002;43:1026–34.
McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45.
Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, et al. Inflammatory markers and poor outcome after stroke: a prospective cohort study and systematic review of interleukin-6. PLoS Med. 2009;6:e1000145.
Jiang L, Chen SH, Chu CH, Wang SJ, Oyarzabal E, Wilson B, et al. A novel role of microglial NADPH oxidase in mediating extra-synaptic function of norepinephrine in regulating brain immune homeostasis. Glia. 2015;63:1057–72.
Rohl C, Lucius R, Sievers J. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res. 2007;1129:43–52.
Dong YF, Chen ZZ, Zhao Z, Yang DD, Yan H, Ji J, et al. Potential role of microRNA-7 in the anti-neuroinflammation effects of nicorandil in astrocytes induced by oxygen-glucose deprivation. J Neuroinflammation. 2016;13:60.
Lana D, Melani A, Pugliese AM, Cipriani S, Nosi D, Pedata F, et al. The neuron-astrocyte-microglia triad in a rat model of chronic cerebral hypoperfusion: protective effect of dipyridamole. Front Aging Neurosci. 2014;6:322.
Ballestas ME, Benveniste EN. Elevation of cyclic AMP levels in astrocytes antagonizes cytokine-induced adhesion molecule expression. J Neurochem. 1997;69:1438–48.
Frohman EM, Vayuvegula B, Gupta S, van den Noort S. Norepinephrine inhibits gamma-interferon-induced major histocompatibility class II (Ia) antigen expression on cultured astrocytes via beta-2-adrenergic signal transduction mechanisms. Proc Natl Acad Sci U S A. 1988;85:1292–6.
Madrigal JL, Caso JR, Garcia-Bueno B, Gutierrez IL, Leza JC. Noradrenaline induces CX3CL1 production and release by neurons. Neuropharmacology. 2017;114:146–55.
Limatola C, Ransohoff RM. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front Cell Neurosci. 2014;8:229.
Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–901.
Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–52.
Hinojosa AE, Caso JR, Garcia-Bueno B, Leza JC, Madrigal JL. Dual effects of noradrenaline on astroglial production of chemokines and pro-inflammatory mediators. J Neuroinflammation. 2013;10:81.
Catalano M, Lauro C, Cipriani R, Chece G, Ponzetta A, Di Angelantonio S, et al. CX3CL1 protects neurons against excitotoxicity enhancing GLT-1 activity on astrocytes. J Neuroimmunol. 2013;263:75–82.
Mallick BN, Adya HV, Faisal M. Norepinephrine-stimulated increase in Na+, K+-ATPase activity in the rat brain is mediated through alpha1A-adrenoceptor possibly by dephosphorylation of the enzyme. J Neurochem. 2000;74:1574–8.
Kim MJ, Hur J, Ham IH, Yang HJ, Kim Y, Park S, et al. Expression and activity of the Na-K ATPase in ischemic injury of primary cultured astrocytes. Korean J Physiol Pharmacol. 2013;17:275–81.
Song M, Yu SP. Ionic regulation of cell volume changes and cell death after ischemic stroke. Transl Stroke Res. 2014;5:17–27.
O’Sullivan JB, Ryan KM, Curtin NM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int J Neuropsychopharmacol. 2009;12:687–99.
O'Sullivan JB, Ryan KM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors inhibit expression of chemokines IP-10 and RANTES and cell adhesion molecules VCAM-1 and ICAM-1 in the CNS following a systemic inflammatory challenge. J Neuroimmunol. 2010;220:34–42.
Simi A, Tsakiri N, Wang P, Rothwell NJ. Interleukin-1 and inflammatory neurodegeneration. Biochem Soc Trans. 2007;35:1122–6.
McNamee EN, Ryan KM, Kilroy D, Connor TJ. Noradrenaline induces IL-1ra and IL-1 type II receptor expression in primary glial cells and protects against IL-1beta-induced neurotoxicity. Eur J Pharmacol. 2010;626:219–28.
McNamee EN, Griffin EW, Ryan KM, Ryan KJ, Heffernan S, Harkin A, et al. Noradrenaline acting at beta-adrenoceptors induces expression of IL-1beta and its negative regulators IL-1ra and IL-1RII, and drives an overall anti-inflammatory phenotype in rat cortex. Neuropharmacology. 2010;59:37–48.
Lundkvist J, Sundgren-Andersson AK, Tingsborg S, Ostlund P, Engfors C, Alheim K, et al. Acute-phase responses in transgenic mice with CNS overexpression of IL-1 receptor antagonist. Am J Phys. 1999;276:R644–51.
Liu J, Zhao ML, Brosnan CF, Lee SC. Expression of type II nitric oxide synthase in primary human astrocytes and microglia: role of IL-1beta and IL-1 receptor antagonist. J Immunol. 1996;157:3569–76.
Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull. 1992;29:243–6.
McNamee EN, Ryan KM, Griffin EW, Gonzalez-Reyes RE, Ryan KJ, Harkin A, et al. Noradrenaline acting at central beta-adrenoceptors induces interleukin-10 and suppressor of cytokine signaling-3 expression in rat brain: implications for neurodegeneration. Brain Behav Immun. 2010;24:660–71.
Ledeboer A, Breve JJ, Wierinckx A, van der Jagt S, Bristow AF, Leysen JE, et al. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur J Neurosci. 2002;16:1175–85.
Park KW, Lee HG, Jin BK, Lee YB. Interleukin-10 endogenously expressed in microglia prevents lipopolysaccharide-induced neurodegeneration in the rat cerebral cortex in vivo. Exp Mol Med. 2007;39:812–9.
Bachis A, Colangelo AM, Vicini S, Doe PP, De Bernardi MA, Brooker G, et al. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci. 2001;21:3104–12.
Gleeson LC, Ryan KJ, Griffin EW, Connor TJ, Harkin A. The beta2-adrenoceptor agonist clenbuterol elicits neuroprotective, anti-inflammatory and neurotrophic actions in the kainic acid model of excitotoxicity. Brain Behav Immun. 2010;24:1354–61.
Culmsee C, Semkova I, Krieglstein J. NGF mediates the neuroprotective effect of the beta2-adrenoceptor agonist clenbuterol in vitro and in vivo: evidence from an NGF-antisense study. Neurochem Int. 1999;35:47–57.
Oshima M, Koizumi S, Fujita K, Guroff G. Nerve growth factor-induced decrease in the calpain activity of PC12 cells. J Biol Chem. 1989;264:20811–6.
Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–85.
Rami A, Volkmann T, Agarwal R, Schoninger S, Nurnberger F, Saido TC, et al. beta2-Adrenergic receptor responsiveness of the calpain-calpastatin system and attenuation of neuronal death in rat hippocampus after transient global ischemia. Neurosci Res. 2003;47:373–82.
Sun M, Xu C. Neuroprotective mechanism of taurine due to up-regulating calpastatin and down-regulating calpain and caspase-3 during focal cerebral ischemia. Cell Mol Neurobiol. 2008;28:593–611.
Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology. 1991;75:328–32.
Gustafson I, Westerberg E, Wieloch T. Protection against ischemia-induced neuronal damage by the alpha 2-adrenoceptor antagonist idazoxan: influence of time of administration and possible mechanisms of action. J Cereb Blood Flow Metab. 1990;10:885–94.
Haller J, Makara GB, Pinter I, Gyertyan I, Egyed A. The mechanism of action of alpha 2 adrenoceptor blockers as revealed by effects on open field locomotion and escape reactions in the shuttle-box. Psychopharmacology. 1997;134:107–14.
Nellgard B, Mackensen GB, Sarraf-Yazdi S, Miura Y, Pearlstein R, Warner DS. Pre-ischemic depletion of brain norepinephrine decreases infarct size in normothermic rats exposed to transient focal cerebral ischemia. Neurosci Lett. 1999;275:167–70.
Nellgard BM, Miura Y, Mackensen GB, Pearlstein RD, Warner DS. Effect of intracerebral norepinephrine depletion on outcome from severe forebrain ischemia in the rat. Brain Res. 1999;847:262–9.
Martinez G, Di Giacomo C, Sorrenti V, Carnazza ML, Bisceglie V, Vanella A. Effects of norepinephrine depletion in rats during cerebral post-ischemic reperfusion. Neurotoxicology. 2004;25:877–84.
Heal DJ, Butler SA, Prow MR, Buckett WR. Quantification of presynaptic alpha 2-adrenoceptors in rat brain after short-term DSP-4 lesioning. Eur J Pharmacol. 1993;249:37–41.
Mogilnicka E. Increase in beta- and alpha 1-adrenoceptor binding sites in the rat brain and in the alpha 1-adrenoceptor functional sensitivity after the DSP-4-induced noradrenergic denervation. Pharmacol Biochem Behav. 1986;25:743–6.
Jonsson G, Hallman H, Sundstrom E. Effects of the noradrenaline neurotoxin DSP4 on the postnatal development of central noradrenaline neurons in the rat. Neuroscience. 1982;7:2895–907.
Peroutka SJ. 5-Hydroxytryptamine receptors. J Neurochem. 1993;60:408–16.
Boyeson MG, Krobert KA. Cerebellar norepinephrine infusions facilitate recovery after sensorimotor cortex injury. Brain Res Bull. 1992;29:435–9.
Boyeson MG, Harmon RL. Effects of trazodone and desipramine on motor recovery in brain-injured rats. Am J Phys Med Rehabil. 1993;72:286–93.
Sutton RL, Feeney DM. Alpha-Noradrenergic agonists and antagonists affect recovery and maintenance of beam-walking ability after sensorimotor cortex ablation in the rat. Restor Neurol Neurosci. 1992;4:1–11.
Goldstein LB, Coviello A, Miller GD, Davis JN. Norepinephrine depletion impairs motor recovery following sensorimotor cortex injury in the rat. Restor Neurol Neurosci. 1991;3:41–7.
Windle V, Power A, Corbett D. Norepinephrine depletion facilitates recovery of function after focal ischemia in the rat. Eur J Neurosci. 2007;26:1822–31.
Fritschy JM, Grzanna R. Restoration of ascending noradrenergic projections by residual locus coeruleus neurons: compensatory response to neurotoxin-induced cell death in the adult rat brain. J Comp Neurol. 1992;321:421–41.
Hughes ZA, Stanford SC. A partial noradrenergic lesion induced by DSP-4 increases extracellular noradrenaline concentration in rat frontal cortex: a microdialysis study in vivo. Psychopharmacology (Berl). 1998;136:299–303.
Goldstein LB, Lennihan L, Rabadi MJ, Good DC, Reding MJ, Dromerick AW, et al. Effect of dextroamphetamine on poststroke motor recovery: a randomized clinical trial. JAMA Neurol. 2018.
Schuster C, Maunz G, Lutz K, Kischka U, Sturzenegger R, Ettlin T. Dexamphetamine improves upper extremity outcome during rehabilitation after stroke: a pilot randomized controlled trial. Neurorehabil Neural Repair. 2011;25:749–55.
Sprigg N, Willmot MR, Gray LJ, Sunderland A, Pomeroy V, Walker M, et al. Amphetamine increases blood pressure and heart rate but has no effect on motor recovery or cerebral haemodynamics in ischaemic stroke: a randomized controlled trial (ISRCTN 36285333). J Hum Hypertens. 2007;21:616–24.
Sonde L, Lokk J. Effects of amphetamine and/or L-dopa and physiotherapy after stroke—a blinded randomized study. A Neurol Scand. 2007;115:55–9.
Sonde L, Lokk J. Effects of amphetamine and/or L-dopa and physiotherapy after stroke—a blinded randomized study. Acta Neurol Scand. 2007;115:55–9.
Platz T, Kim IH, Engel U, Pinkowski C, Eickhof C, Kutzner M. Amphetamine fails to facilitate motor performance and to enhance motor recovery among stroke patients with mild arm paresis: interim analysis and termination of a double blind, randomised, placebo-controlled trial. Restor Neurol Neurosci. 2005;23:271–80.
Gladstone DJ, Danells CJ, Armesto A, McIlroy WE, Staines WR, Graham SJ, et al. Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. 2006;37:179–85.
Martinsson L, Wahlgren NG. Safety of dexamphetamine in acute ischemic stroke: a randomized, double-blind, controlled dose-escalation trial. Stroke. 2003;34:475–81.
Martinsson L, Eksborg S, Wahlgren NG. Intensive early physiotherapy combined with dexamphetamine treatment in severe stroke: a randomized, controlled pilot study. Cerebrovasc Dis. 2003;16:338–45.
Treig T, Werner C, Sachse M, Hesse S. No benefit from D-amphetamine when added to physiotherapy after stroke: a randomized, placebo-controlled study. Clin Rehabil. 2003;17:590–9.
Walker-Batson D, Smith P, Curtis S, Unwin H, Greenlee R. Amphetamine paired with physical therapy accelerates motor recovery after stroke. Further evidence. Stroke. 1995;26:2254–9.
Crisostomo EA, Duncan PW, Propst M, Dawson DV, Davis JN. Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann Neurol. 1988;23:94–7.
Zittel S, Weiller C, Liepert J. Reboxetine improves motor function in chronic stroke. A pilot study. J Neurol. 2007;254:197–201.
Walker-Batson D, Curtis S, Natarajan R, Ford J, Dronkers N, Salmeron E, et al. A double-blind, placebo-controlled study of the use of amphetamine in the treatment of aphasia. Stroke. 2001;32:2093–8.
Keser Z, Dehgan MW, Shadravan S, Yozbatiran N, Maher LM, Francisco GE. Combined dextroamphetamine and transcranial direct current stimulation in poststroke aphasia. Am J Phys Med Rehabil. 2017;96:S141–S5.
Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of induced hypertension on intracranial pressure and flow velocities of the middle cerebral arteries in patients with large hemispheric stroke. Stroke. 2002;33:998–1004.
Rordorf G, Koroshetz WJ, Ezzeddine MA, Segal AZ, Buonanno FS. A pilot study of drug-induced hypertension for treatment of acute stroke. Neurology. 2001;56:1210–3.
Marzan AS, Hungerbuhler HJ, Studer A, Baumgartner RW, Georgiadis D. Feasibility and safety of norepinephrine-induced arterial hypertension in acute ischemic stroke. Neurology. 2004;62:1193–5.
Hillis AE, Ulatowski JA, Barker PB, Torbey M, Ziai W, Beauchamp NJ, et al. A pilot randomized trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovasc Dis. 2003;16:236–46.
Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–10.
Collin C, Wade DT, Davies S, Horne V. The Barthel ADL index: a reliability study. Int Disabil Stud. 1988;10:61–3.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–7.
Ver Hage A. The NIH stroke scale: a window into neurological status. Nurs Spectr. 2011;24:44–9.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Multicenter trial of hemodilution in ischemic stroke—background and study protocol. Scandinavian Stroke Study Group. Stroke. 1985;16:885–90.
Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, et al. Measuring physical impairment and disability with the Chedoke-McMaster stroke assessment. Stroke. 1993;24:58–63.
Van de Winckel A, Feys H, Lincoln N, De Weerdt W. Assessment of arm function in stroke patients: Rivermead Motor Assessment arm section revised with Rasch analysis. Clin Rehabil. 2007;21:471–9.
Porch BE. The Porch Index of Communicative Abilities. Palo Alto: Consulting Psychologists Press; 1982.
Shewan CM, Kertesz A. Reliability and validity characteristics of the Western Aphasia Battery (WAB). J Speech Hear Disord. 1980;45:308–24.
Schwarz AJ, Gozzi A, Reese T, Heidbreder CA, Bifone A. Pharmacological modulation of functional connectivity: the correlation structure underlying the phMRI response to d-amphetamine modified by selective dopamine D3 receptor antagonist SB277011A. Magn Reson Imaging. 2007;25:811–20.
Berman SM, Kuczenski R, McCracken JT, London ED. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol Psychiatry. 2009;14:123–42.
Turowski P, Kenny BA. The blood-brain barrier and methamphetamine: open sesame? Front Neurosci. 2015;9:156.
Neumann-Haefelin T, Wittsack HJ, Wenserski F, Siebler M, Seitz RJ, Modder U, et al. Diffusion- and perfusion-weighted MRI. The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30:1591–7.
Bevan JA, Duckworth J, Laher I, Oriowo MA, McPherson GA, Bevan RD. Sympathetic control of cerebral arteries: specialization in receptor type, reserve, affinity, and distribution. FASEB J. 1987;1:193–8.
Qureshi AI, Ezzeddine MA, Nasar A, Suri MF, Kirmani JF, Hussein HM, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med. 2007;25:32–8.
Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, et al. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Stroke. 2009;40:2442–9.
Hatashita S, Hoff JT, Ishii S. Focal brain edema associated with acute arterial hypertension. J Neurosurg. 1986;64:643–9.
Pfister D, Strebel SP, Steiner LA. Effects of catecholamines on cerebral blood vessels in patients with traumatic brain injury. Eur J Anaesthesiol Suppl. 2008;42:98–103.
del Carmen Garcia M, Enero MA, Celuch SM. Hypotensive and hypertensive effects of catecholamines intrathecally injected in anesthetized rats. J Auton Nerv Syst. 1996;59:17–26.
Krishna B, Hussain ME, Chakrabarty AS, Jain AK, Chakrabarty K, Fahim M. Hypotensive effect of intracerebroventricular injection of norepinephrine and its modulation by alpha and beta adrenergic blockers in conscious rabbits. Indian J Physiol Pharmacol. 1995;39:361–8.
Day MD, Roach AG. Central alpha- and beta-adrenoceptors modifying arterial blood pressure and heart rate in conscious cats. Br J Pharmacol. 1974;51:325–33.
Beal AM, Bligh J. Diuretic effect of intraventricular and intravenous infusions of noradrenaline in conscious sheep. Q J Exp Physiol Cogn Med Sci. 1980;65:321–33.
Bekar LK, Wei HS, Nedergaard M. The locus coeruleus-norepinephrine network optimizes coupling of cerebral blood volume with oxygen demand. J Cereb Blood Flow Metab. 2012;32:2135–45.
Armstead WM, Riley J, Vavilala MS. Norepinephrine protects cerebral autoregulation and reduces hippocampal necrosis after traumatic brain injury via blockade of ERK MAPK and IL-6 in juvenile pigs. J Neurotrauma. 2016;33:1761–7.
Sandu N, Spiriev T, Lemaitre F, Filis A, Schaller B, Trigemino G. Cardiac reflex examination, new molecular knowledge towards the trigemino-cardiac reflex as a cerebral oxygen-conserving reflex. ScientificWorldJournal. 2010;10:811–7.
Schaller B, Cornelius JF, Sandu N, Ottaviani G, Perez-Pinzon MA. Oxygen-conserving reflexes of the brain: the current molecular knowledge. J Cell Mol Med. 2009;13:644–7.
Imani A, Faghihi M, Sadr SS, Niaraki SS, Alizadeh AM. Noradrenaline protects in vivo rat heart against infarction and ventricular arrhythmias via nitric oxide and reactive oxygen species. J Surg Res. 2011;169:9–15.
Ravingerova T, Pancza D, Ziegelhoffer A, Styk J. Preconditioning modulates susceptibility to ischemia-induced arrhythmias in the rat heart: the role of alpha-adrenergic stimulation and K(ATP) channels. Physiol Res. 2002;51:109–19.
Ni Chroinin D, Asplund K, Asberg S, Callaly E, Cuadrado-Godia E, Diez-Tejedor E, et al. Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44:448–56.
Cho KJ, Cheon SY, Kim GW. Statins promote long-term recovery after ischemic stroke by reconnecting noradrenergic neuronal circuitry. Neural Plast. 2015;2015:585783.
Zhang G, Chen L, Yang L, Hua X, Zhou B, Miao Z, et al. Combined use of spatial restraint stress and middle cerebral artery occlusion is a novel model of post-stroke depression in mice. Sci Rep. 2015;5:16751.
Chen B, Zhang Y, Chen L, Huang S, Li S, Yao J. Dose-effects of aorta-infused clenbuterol on spinal cord ischemia-reperfusion injury in rabbits. PLoS One. 2013;8:e84095.
Culmsee C, Junker V, Kremers W, Thal S, Plesnila N, Krieglstein J. Combination therapy in ischemic stroke: synergistic neuroprotective effects of memantine and clenbuterol. Stroke. 2004;35:1197–202.
Culmsee C, Junker V, Thal S, Kremers W, Maier S, Schneider HJ, et al. Enantio-selective effects of clenbuterol in cultured neurons and astrocytes, and in a mouse model of cerebral ischemia. Eur J Pharmacol. 2007;575:57–65.
Murugaiah KD, O’Donnell JM. Clenbuterol increases norepinephrine release from rat brain slices by a calcium- and receptor-independent mechanism. Res Commun Mol Pathol Pharmacol. 1994;86:311–24.
Vonck K, Raedt R, Naulaerts J, De Vogelaere F, Thiery E, Van Roost D, et al. Vagus nerve stimulation…25 years later! What do we know about the effects on cognition? Neurosci Biobehav Rev. 2014;45:63–71.
Kreuzer PM, Landgrebe M, Husser O, Resch M, Schecklmann M, Geisreiter F, et al. Transcutaneous vagus nerve stimulation: retrospective assessment of cardiac safety in a pilot study. Front Psychiatry. 2012;3:70.
Van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol. 1999;412:410–28.
George MS, Aston-Jones G. Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology. 2010;35:301–16.
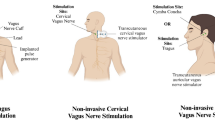
Redgrave JN, Moore L, Oyekunle T, Ebrahim M, Falidas K, Snowdon N, et al. Transcutaneous auricular vagus nerve stimulation with concurrent upper limb repetitive task practice for poststroke motor recovery: a pilot study. J Stroke Cerebrovasc Dis. 2018;27:1998–2005.
Capone F, Miccinilli S, Pellegrino G, Zollo L, Simonetti D, Bressi F, et al. Transcutaneous vagus nerve stimulation combined with robotic rehabilitation improves upper limb function after stroke. Neural Plast. 2017;2017:7876507.
Liu AF, Zhao FB, Wang J, Lu YF, Tian J, Zhao Y, et al. Effects of vagus nerve stimulation on cognitive functioning in rats with cerebral ischemia reperfusion. J Transl Med. 2016;14:101.
Chiluwal A, Narayan RK, Chaung W, Mehan N, Wang P, Bouton CE, et al. Neuroprotective effects of trigeminal nerve stimulation in severe traumatic brain injury. Sci Rep. 2017;7:6792.
Kroppenstedt SN, Thomale UW, Griebenow M, Sakowitz OW, Schaser KD, Mayr PS, et al. Effects of early and late intravenous norepinephrine infusion on cerebral perfusion, microcirculation, brain-tissue oxygenation, and edema formation in brain-injured rats. Crit Care Med. 2003;31:2211–21.
Boyeson MG, Feeney DM. Intraventricular norepinephrine facilitates motor recovery following sensorimotor cortex injury. Pharmacol Biochem Behav. 1990;35:497–501.
Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–36.
Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–39.
Martin NA, Patwardhan RV, Alexander MJ, Africk CZ, Lee JH, Shalmon E, et al. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87:9–19.
Northoff G, Heiss WD. Why is the distinction between neural predispositions, prerequisites, and correlates of the level of consciousness clinically relevant?: functional brain imaging in coma and vegetative state. Stroke. 2015;46:1147–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sternberg, Z., Schaller, B. Central Noradrenergic Agonists in the Treatment of Ischemic Stroke—an Overview. Transl. Stroke Res. 11, 165–184 (2020). https://doi.org/10.1007/s12975-019-00718-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-019-00718-7