Abstract
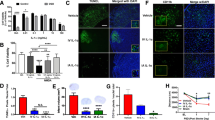
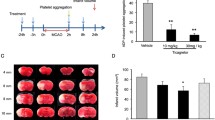
The sole FDA-approved drug treatment for ischemic stroke is tissue-type plasminogen activator (tPA). However, upregulation of JNK mitogen-activated protein kinase (MAPK) and endothelin 1 (ET-1) by tPA after stroke contributes to impaired cerebrovascular autoregulation. Wild-type (wt) tPA can bind to the lipoprotein-related receptor (LRP), which mediates vasodilation, or NMDA receptors (NMDA-Rs), exacerbating vasoconstriction. Elevations in IL-6, a marker of inflammation that accompanies stroke, are reported to be an adverse prognostic factor. We hypothesized that IL-6 released into CSF after stroke by wt-tPA through activation of NMDA-Rs and upregulation of ET-1 and JNK contribute to impairment of cerebrovascular autoregulation and increased histopathology. Results show that IL-6 was increased post stroke in pigs, which was increased further by wt-tPA. Co-administration of the IL-6 antagonist LMT-28 with wt-tPA prevented impairment of cerebrovascular autoregulation and necrosis of hippocampal cells. wt-tPA co-administered with the JNK inhibitor SP 600125 and the ET-1 antagonist BQ 123 blocked stroke-induced elevation of IL-6. Co-administration of LMT-28 with wt-tPA blocked the augmentation of JNK and ET-1 post stroke. In conclusion, IL-6 released after stroke, which is enhanced by wt-tPA through activation of NMDA-Rs and upregulation of ET-1 and JNK, impairs cerebrovascular autoregulation and increases histopathology. Strategies that promote fibrinolysis while limiting activation of NMDA-Rs and upregulation of IL-6 may improve the benefit/risk ratio compared to wt-tPA in treatment of stroke.





Similar content being viewed by others
References
Freeman SS, Udomphorn Y, Armstead WM, Fisk DM, Vavilala MS. Young age as a risk factor for impaired cerebral autoregulation after moderate-severe pediatric brain injury. Anesthesiology. 2008;108:588–95.
Lapchak PA. Hemorrhagic transformation following ischemic stroke: significance, causes and relationship to therapy and treatment. Curr Neurol Neurosci Rep. 2002;2:1–6.
Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–76.
Lipton P. Ischemic cell death in brain neurons. Physiolog Rev. 1999;79:1431–568.
Armstead WM. NOC/oFQ and NMDA contribute to piglet hypoxic ischemic hypotensive cerebrovasodilation impairment. Ped Res. 2002;51:586–91.
Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97.
Park L, Gallo EF, Anrather J, Wang G, Norris EH, Paul J, et al. Key role of tissue plasminogen activator in neurovascular coupling. Proc Natl Acad Sci. 2008;105:1073–8.
Armstead WM. Age dependent NMDA contribution to impaired hypotensive cerebral hemodynamics following brain injury. Develop Brain Res. 2002;139:19–28.
Collingridge G, Lester R. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989;41:143–210.
Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on michondrial function. Neuron. 1995;15:961–73.
Armstead WM, Riley J, Kiessling JW, Cines DB, Higazi AAR. Novel plasminogen activator inhibitor-1 derived peptide protects against impairment of cerebrovasodilation after photothrombosis through inhibition of JNK MAPK. Am J Phys. 2010;299:R480–5.
Armstead WM, Riley J, Yarovoi S, Higazi AAR, Cines DB. Tissue type plasminogen activator–A296-299 prevents impairment of cerebral autoregulation after stroke through lipoprotein-related receptor-dependent increase in cAMP and p38. Stroke. 2016;47:2096–102.
Murray KN, Parry-Jones AR, Allan SM. Interleukin-1 and brain injury. Front Cell Neurol. 2015;9:1–13.
Armstead WM, Riley J, Vavilala MS. Norepinephrine protects autoregulation and reduces hippocampal necrosis after traumatic brain injury via block of ERK MAPK and IL-6 in juvenile pigs. J Neurotrauma. 2016;33:1761–7.
Dieplinger B, Bocksrucker C, Egger M, Eggers C, Haltmayer M, Mueller T. Prognostic value of inflammatory and cardiovascular biomarkers for prediction of 90 day all-cause mortality after acute ischemic stroke—results from the Linz stroke unit study. Clin Chemistry. 2017;63:1101–9.
Wytrykowska A, Prosba-Mackiewicz M, Nyka WM. IL-1B, TNFa, and IL-6 levels in gingival fluid and serum of patients with ischemic stroke. J Oral Science. 2016;58:509–13.
Froyshov HM, Bjornerem A, Engstad T, Halvorsen DS. Elevated inflammatory markers predict mortality in long-term ischemic stroke-survivors: a population-based prospective study. Aging-Clinical and Experimental Research. 2017;29:379–85.
Nassar T, Yarovoi S, Abu Fanne R, Akkawi S, Jammal M, Allen T, et al. Regulation of airway contractility by plasminogen activators through NMDA receptor-1. Am J Respr Cell Molec Biol. 2010;43:703–11.
Armstead WM, Hekierski H, Yarovoi S, Higazi AAR, Cines DB. tPA-A296-299 prevents impairment of cerebral autoregulation and necrosis of hippocampal neurons after stroke by inhibiting upregulation of ET-1. J of Neurosci Res. 2017;96:128–37.
Kuluz JW, Prado R, He D, Zhao W, Dietrich WD, Watson B. New pediatric model of ischemic stroke in infant piglets by photothrombosis. Stroke. 2007;38:1932–7.
Hong SS, Choi JH, Lee SY, Park YH, Park KY, Lee JY, et al. Novel small-molecule inhibitor targeting the IL-receptor B subunit, glycoprotein 130. J Immunol. 2015;195:237–45.
Yao X, Huang H, Zhong F, Shen N, Faggioni R, Fung M, et al. Targeting interleukin 6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125–39.
Armstead WM, Ganguly K, Kiessling JW, Riley J, Chen XH, Smith DH, et al. Signaling, delivery, and age as emerging issues in the benefit/risk ratio outcome of tPA for treatment of CNS ischemic disorders. J Neurochemistry. 2010;113:303–12.
Girling KJ, Cavill G, Mahajan RP. The effects of nitrous oxide and oxygen consumption on transient hyperemic response in human volunteers. Anesth Analg. 1999;89:175–80.
Armstead WM, Riley J, Vavilala MS. Sex and age differences in epinephrine mechanisms and outcomes after brain injury. J Neurotrauma. 2017;34:1666–75.
Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150–8.
Armstead WM, Ganguly K, Riley J, Kiessling JW, Cines DB, Higazi AAR, et al. RBC-coupled tPA prevents impairment of cerebral vasodilatory responses through inhibition of JNK and potentiation of p38 MAPK after cerebral photothrombosis. Ped. Crit Care Med. 2011;12:369–75.
Armstead WM, Kiessling JW, Riley J, Cines DB, Higazi AAR. tPA contributes to impaired NMDA cerebrovasodilation after traumatic brain injury through activation of JNK MAPK. Neurol Res. 2011;33:726–33.
Armstead WM. Endothelins and the role of endothelin antagonists in the management of posttraumatic vasospasm. Curr Pharm Des. 2004;10:2185–92.
Choi EK, Yeo JS, Park CY, Na HI, Lim JA, Lee JE, et al. Inhibition of reactive oxygen species downregulates the MAPK pathway in rat spinal cord after limb ischemia reperfusion injury. Int Journal of Surgery. 2015;22:74–8.
Ross J, Armstead WM. Differential role of PTK and ERK MAPK in superoxide impairment of Katp and Kca channel cerebrovasodilation. AJP. 2003;285:R149–54.
Shin HK, Lee JH, Kim KY, Kim CD, Lee WS, Rhim BY, et al. Impairment of autoregulatory vasodilation by NADPH oxidase-dependent superoxide generation during acute stage of subarachnoid hemorrhage in rat pial artery. JCBFM. 2002;22:869–77.
Funding
This study was funded by grants from the National Institutes of Health, NS090998 (WMA), HL76406, CA83121, HL76206, HL07971, and HL81864 (DBC), HL77760 and HL82545 (AARH), and the Israeli Science Foundation (AARH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Armstead, W.M., Hekierski, H., Pastor, P. et al. Release of IL-6 After Stroke Contributes to Impaired Cerebral Autoregulation and Hippocampal Neuronal Necrosis Through NMDA Receptor Activation and Upregulation of ET-1 and JNK. Transl. Stroke Res. 10, 104–111 (2019). https://doi.org/10.1007/s12975-018-0617-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-018-0617-z