Abstract
Genetic counselling and testing are essential health services for the management of heritable diseases. However, in low-and-middle income countries like Kenya, genetic counsellors are not yet a licenced profession, and there is limited availability of and access to genetic testing. This study aimed to uncover opportunities and barriers for genetic service delivery in the Kenyan healthcare system from the perspectives of those who provide genetic testing and/or genetic counselling. Participants included Kenyan health personnel who deliver genetic services. This was a qualitative study that collected data via semi-structured one-on-one interviews and analyzed it using inductive thematic analysis. Participant demographics and characteristics of clinical genetic service provision were collected using a survey and results summarized using descriptive statistics. Themes revealed during analysis were compared to the clinical characteristics of genetic service provision to inform the opportunities and barriers. Fifteen interviews were conducted in total. Thematic analysis indicated that participants believed that the barriers facing genetic service delivery were linked to three themes: (1) education and training, (2) costs, and (3) counselling challenges. The opportunities for genetic service delivery were linked to four themes: (1) demand, (2) education and training, (3) encouraging a multidisciplinary approach to care, and (4) enhancing laboratory infrastructure. These findings are crucial for the development of a national evidence-informed and culturally appropriate model for genetic service delivery.
Similar content being viewed by others
Introduction
The delivery of clinical genetic services, such as genetic testing and counselling, is crucial for the effective management of heritable disorders, diseases, and syndromes. Genetic testing can be broadly defined as the analysis of human DNA, RNA, proteins, and metabolites in order to identify the aberrant genotypes (mutations) or molecular phenotypes characteristic of a heritable disease (Genetics Home Reference 2019; McPherson 2006). Genetic screening of at-risk individuals can rule out or confirm the presence of a genetic condition, identify carriers, establish a diagnosis, and be used to predict the risk of disease for clinical prognosis (Burke 2002). Genetic counselling is a health communication practice that helps people “understand and adapt to the medical, psychological and familial implications of genetic contributions to disease” (Resta et al. 2006). Genetic counsellors are certified health professionals who have completed specialized training in medical genetics and counselling (Canadian Association of Genetic Counsellors 2021).
Previous research has shown that the capacity, accessibility, delivery, and quality of genetic services vary widely across low-and-middle-income countries (LMICs) (Hawkins and Hayden 2011; He et al. 2014; Zhong et al. 2018). There are many challenges when delivering genetic services in LMICs—such as the need for more efficient service delivery methods, as well as limitations in human, technical, and financial resources. These challenges result in the insufficient or complete absence of genetic services and arguably require significant investment to meet the growing disease burden (Trepanier and Allain 2014; Wonkam et al. 2011).
The global genetic disease burden in newborns, when followed until the age of 25, is approximately 5.32% (Verma and Puri 2015). At least 7.6 million infants are born annually with genetic or congenital conditions, of which 90% reside in LMICs (World Health Organization 2005). In Kenya, patients have little-to-no access to genetic testing and counselling services, as documented in a study of physician experience in managing retinoblastoma patients (He et al. 2014). In the absence of licensed genetic counsellors (Ormond et al. 2018), patients are counselled to the best of their abilities by the managing physician, who often have variable training in genetics (He et al. 2014). Patients with heritable cancers in Kenya have expressed a desire for improved access to counselling and more comprehensive genetic testing services, recognizing the benefits of early diagnosis and medical management of their condition (Gedleh et al. 2018; Lee et al. 2017). Furthermore, it is important to address the societal and cultural implications of sharing genetic information about heritable conditions which often have an associated stigma attached, as shown through a study of sickle cell disease in Kenya (Marsh et al. 2010, 2013).
In recent years, novel genetic technology has become more available in LMICs through research initiatives (H3Africa Consortium 2014; Nordling 2017). In Kenya, clinical genetic testing in the public sector is limited, and some providers have started to appear in the private sector, but their relevance, quality, accessibility, and uptake have not been examined. Additional context-specific investigation is required to reveal factors that facilitate delivery and uptake of quality genetic services. It is equally important to understand these factors from the viewpoint of the individuals delivering genetic services, as their intimate understanding of the landscape can provide researchers and policy-makers with valuable insight on the healthcare sector. In order to understand these perspectives, we conducted a qualitative study with Kenyan health personnel (HPs) who deliver genetic services (i.e., genetic counselling, genetic testing) in order to elucidate the perceived opportunities and barriers of genetic service delivery in Kenya.
Methods
Study design
This was a qualitative study that aimed to discover and explain the process of genetic service delivery and its associated opportunities and barriers through the insights and perspectives of HPs. Research ethics approval was obtained from the University of Toronto and the University of Nairobi.
Inclusion and exclusion criteria
Participants were restricted to English-speaking HPs in Kenya who counseled patients with genetic diseases or disorders, and/or provided genetic testing services; these included, but were not limited to, physicians, nurses, and medical laboratory personnel. Participants who did not explicitly fulfill these criteria or declined to provide informed consent were excluded from the study.
Participant recruitment
The study used a combination of purposive sampling strategies to identify and recruit participants. Using publicly available data and the study investigators’ (LN, HD) personal knowledge of the clinical landscape in Kenya, an initial list of potential participants was generated. Researchers used a snowball sampling strategy from this point forward, generating new contacts from the suggestions of enrolled participants. The snowball sampling strategy was also used to facilitate theoretical sampling—whereby recruitment of participants is centered around the development of the emerging theory.
Data collection
After obtaining written, informed consent, participants were asked to complete a paper questionnaire (Supplementary File 1). The survey was self-directed, and the researchers on-site were available to clarify any questions or misunderstandings if requested. The outcomes of interest were primarily categorical data and binary data, which included clinical characteristics of genetic service delivery from both the genetic counselling and genetic testing perspectives.
Following the questionnaire, semi-structured, in-depth interviews were conducted face-to-face, primarily in a one-on-one environment. Using an interview guide developed by the study team (Supplementary File 2), the researcher conducting the interview posed the questions and clarified any misunderstandings where necessary. The semi-structured format allowed participants to share their thoughts with no constraints and provided an opportunity for the researcher to probe for further details. The interview was recorded with the participant’s consent. Where possible, one note-taker was also present in the room to capture written data, complementing the audio recordings. Interviews were conducted until a saturation of themes was reached. The interview lasted approximately 45–60 min.
Data analysis
Information collected from the questionnaire was transferred into Microsoft Excel for data management and statistical analysis. Missing data was identified at this stage and filled in manually by following up with participants, where possible. Descriptive statistics were calculated for all response variables to show the distribution, variability, and trends in the responses across different HP occupations.
Interview recordings were transcribed verbatim by the researchers. Study participants’ names were de-identified and assigned numerical study number identifiers. Thematic analysis of the interview was performed using an inductive and data-driven process. NVIVO 12 was used as the primary data management software. A codebook was created by analyzing the first interview. As additional interviews were conducted, the codebook was expanded and refined. The most basic elements of the raw data were coded first; then relevant codes were grouped together into broader categories. The prevalence of each theme among all the interviews was noted. Direct quotes from the interviews that were illustrative of the themes were chosen to enhance the analysis.
Results
Study participant demographics
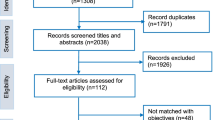
Twenty-four individuals were identified and invited to participate in interviews. Sixteen individuals agreed to participate in the study; the rest either did not respond to the invitation or indicated that they did not have the time. However, only fifteen interviews (15/24, 62.5% response rate) were able to be arranged and conducted between June 2017 and August 2018.
The proportion of male (7/15, 47%) to female (8/15, 53%) participants was balanced (Table 1). The majority of participants possessed advanced degrees holding either an MD or equivalent degree (8/15, 53%) or a PhD or equivalent degree (3/15, 20%) (Table 1). No participant was a certified medical geneticist or certified genetic counsellor (Table 1). Most participants had some form of training in genetics (12/15, 80%), ranging from specialist degrees in genetics (4/15, 27%) to some university courses in genetics (8/15, 53%) (Table 1). The median number of years in practice was 11 (range 4–40), with 80% practicing at a public institution (Table 1).
Genetic counselling
Ten participants indicated that they provided some form of genetic counselling to patients (10/15, 67%). Counselling was mainly for patients with blood disorders (6/10, 60%), heritable cancer (5/10, 50%), and chromosomal disorders (5/10, 50%). Seventy percent (70%) of participants indicated that their counselling was informed by genetic testing reports (Table 2). The subject of counselling sessions was predominantly children (5/10, 50%), with pre-natal (3/10, 30%) and adult (3/10, 30%) cases ranking second (Table 2). Counselling sessions varied in terms of length of duration and provision of resources to patients (Table 2).
Genetic testing
Eleven participants indicated that they offered some type of genetic testing (11/15, 73%). Genetic testing was most commonly offered for chromosomal disorders (9/11, 82%), metabolic disorders (9/11, 82%), and cancer (9/11, 82%) (Table 3). Testing was not uniformly performed in-house, with participants reporting several tests or techniques were outsourced to international laboratories (Table 3).
Themes from interviews
All fifteen participants participated in the semi-structured in-depth interviews. Discussions centered around the participants’ opinions on the barriers and opportunities for genetic service delivery in the Kenyan healthcare setting. The barriers facing genetic service delivery were linked to three main themes: (1) education and training, (2) costs, and (3) counselling challenges (Table 4). The opportunities for genetic service delivery were linked to four themes: (1) demand, (2) education and training, (3) encouraging a multidisciplinary approach to care, and (4) enhancing laboratory infrastructure (Table 5).
Barriers to genetic service delivery
-
1:
Education and training (8/15 interviews)
-
1.1:
Current state of genetics knowledge is limited (8/15 interviews)
When asked if their training and background in genetics had prepared them adequately for their current position, participants referenced that despite completing university level courses in genetics, they lacked the practical expertise to conduct and interpret genetic tests. Some participants went further, indicating that clinicians needed more knowledge about what the genetic tests actually entailed (Table 4: 1.1).
Participants indicated that the curriculum for those working in the Kenyan genetics sector was not suited for the tasks they were performing. Participants regarded this gap in knowledge as a result of their education’s cursory emphasis on genetics (Table 4: 1.1). In some cases, participants spoke about how they had received informal genetics training and that it was their responsibility to seek out the information they needed on their own.
-
1.2:
Continuing professional development opportunities are limited (7/15 interviews)
When asked about resources to expand their professional skills, knowledge, and training, participants indicated that they consumed the majority of their information from an online source due to lack of local availability (Table 4: 1.2). Furthermore, while some participants had sought additional training through fellowships and conferences, these opportunities were not located in Kenya and required participants to travel abroad. Participants indicated that the institutions that they worked at provided some refreshment training for clinicians, but many still face limitations due to the scarcity of these opportunities.
-
1.3:
Lack of practice guidelines (6/15 interviews)
Participants discussed the lack of guidelines for medical genetics as a significant barrier preventing consistent genetic service delivery. Participants expressed the view that regulations and practical guidelines have not been developed because of the relatively low utilization of genetic testing in Kenya. In most cases, participants said that they had to set their own standards. One participant expanded on their experiences with genetic testing and explained that they obtained knowledge through trial and error, as well as by consulting other professionals, given the absence of practice guidelines (Table 4: 1.3). They went on to explain without established guidelines there remained no consequences for error. Participants also explained that genetic testing laboratories are perceived to be equivalent to typical medical diagnostic laboratories and are often forced to follow medical laboratory guidelines despite having distinct operational and ethical considerations to address.
-
1.1:
-
2:
Costs (11/15 interviews)
-
2.1:
High cost of genetic testing (11/15 interviews)
Participants indicated that a major barrier facing patients seeking some sort of genetic testing was the high cost of the tests, as they were mainly available at private healthcare institutions (Table 4: 2.1). Participants identified that the majority of requests and referrals for genetic testing came from the private sector, not the public sector. Participants indicated that for some genetic tests, outside organizations or donors provided the funds necessary for patients to receive necessary diagnostic tests. However, this was not common, and when funds are not available, patients cannot access testing.
-
2.2:
Lack of insurance coverage (9/15 interviews)
In conjunction with the high cost of genetic testing, a major barrier that participants identified was the lack of insurance coverage for genetic tests. Participants indicated that the National Health Insurance Fund (NHIF) did not cover genetic testing (Table 4: 2.2). While some patients reportedly had access to private insurance, whether or not this private insurance actually covered genetic testing was variable. More often than not, participants identified that if patients did have insurance that covered genetic testing, it was likely from an international company. Due to the limited demand for genetic testing by patients, local insurance companies were not covering genetic testing (Table 4: 2.2). Without insurance, patients are left to pay for genetic testing out of pocket. Due to the cost of genetic testing, this makes getting essential tests very challenging—if not impossible—for patients.
-
2.3:
Laboratory capacity limitations lead to outsourcing, raising costs (6/15 interviews)
Limited local capacity to perform genetic testing was highlighted by many participants. Many tests were outsourced to international laboratories, a factor which was perceived to raise costs of testing (Table 4: 2.3). There were a variety of laboratories and countries to which genetic tests were outsourced (Table 4: 2.3). Participants further expressed that one of the major challenges with internationally outsourcing genetic testing was the long turn-around time, which can contribute negatively to patient outcomes. Participants elaborated that while they could be doing more genetic testing than they are currently, they felt held back by the relatively few numbers of tests requested of them. With limited patient demand, it made justifying buying expensive equipment for testing very difficult. Participants indicated that low awareness of genetic testing was a barrier to increasing demand for testing.
-
2.1:
-
3:
Counselling challenges (8/15 interviews)
-
3.1:
Challenges in explaining genetics in non-technical terms (3/15 interviews)
Participants explained that when describing the purpose and results of genetic tests, communicating in terms that the patient would understand remained challenging. Participants explained that patients often have gaps in genetic knowledge, and thus cannot understand what the clinician is trying to convey. Participants also described that genetic testing and counselling inherently involve technical terminology, which cannot always be expressed in non-technical terms, and further create a barrier to patient understanding (Table 4: 3.1).
-
3.2:
Addressing social, cultural, and psychological implications of diagnosis (5/15 interviews)
Participants described difficulty in helping patients address the social stigma associated with genetic disease in Kenya. This resulted in clinicians being reluctant to fully disclose genetic testing results for fear of instigating negative effects, for example, placing strain on the relationship between parents of children with heritable conditions (Table 4: 3.2). Participants indicated that cultural barriers, such as the belief that certain illnesses could be due to witchcraft, could limit patient understanding of genetic testing results. Additionally, participants suggested that much of the stigma surrounding genetic diseases stemmed from the traditional beliefs many families held about the origin of diseases—and how the hospital was often viewed as a last resort for treatment (Table 4: 3.2). Participants further explained that it was not uncommon for patients to doubt the genetic diagnosis and would either abandon treatment or delay follow-up following the delivery of test results.
-
3.3:
Language barriers (4/15 interviews)
Participants spoke at length about the problems stemming from communicating genetic information to patients representing a variety of tribes and ethnicities, as Kenya’s diverse population has many different regional languages (Table 4: 3.3). English was the primary language that participants used to communicate genetic information. In the cases where patients had not completed schooling or did not understand English, communicating genetic information was challenging (Table 4: D3.3).
-
3.1:
Opportunities for genetic service delivery
-
1:
Demand (9/15 interviews)
-
1.1:
Increased public awareness leading to more demand for tests (n = 8 interviews)
Participants indicated that patients were requesting genetic tests more frequently than in the past. This phenomenon was believed to be due to better public understanding of the need for and purpose of genetic testing (Table 5: 1.1). One participant also suggested that increased awareness of and capacity for genetic testing to inform treatment decisions, giving patients access to targeted therapies that were previously unavailable, has contributed to this increased demand (Table 5: 1.1).
Participants also suggested that the public had become increasingly aware of genetic testing (Table 5: 1.1), leading them to believe that demand would likely increase in the near future, driving test availability at an affordable price point. Participants believed that the current cost of genetic testing was a significant barrier to access, but that public awareness might prompt organizations to meet that demand. Furthermore, participants believed that increasing awareness, and thus patients seeking out genetic services sooner, would incentivize the healthcare workforce to further establish local capacity and expertise in genetic testing and counselling.
-
1.2:
Medical utility of genetic testing is increasing (3/15 interviews)
Participants explained that they believed that increased demand for genetic testing due to the utility of technology beyond diagnosis of heritable disorders, for example, in identification of biomarkers for targeted cancer therapy. One participant indicated that this was the case for chronic myeloid leukemia (CML) (Table 5: 1.2); however, they also indicated that this was tied to the fact that the therapy was made available through a collaboration with an NGO. Therefore, medical utility of genetic testing with respect to biomarker detection appeared to be tied to availability of and access to downstream therapies.
-
1.1:
-
2:
Education and training (8/15 interviews)
-
2.1:
Enhance genetic education opportunities (6/15 interviews)
Despite of limited opportunities offered by institutions, participants noted that better education for HPs working in genetic service delivery was needed. Training in genetics or genetic counselling is not a standard part of the medical curriculum, and a larger emphasis on genetics training is required at all levels of the healthcare system. Specifically, participants spoke about the need to incorporate genetics training in the medical curriculum for physicians, medical students, nurses, and laboratory scientists (Table 5: 2.1). One participant iterated how genetic testing and counselling is still perceived as foreign in Kenya (Table 5: 2.1). A greater appreciation of the role genetic counsellors can play in disease management was suggested to be a necessary prerequisite before the role becomes integrated into the healthcare system.
-
2.1:
-
3:
Encourage multidisciplinary approach to care (3/15 interviews)
Participants indicated during their interviews that an opportunity for growth in genetic service delivery was to implement effective communication between genetic testing facilities (e.g., laboratory directors) and counsellors (e.g., physicians) (Table 5: 3).
-
4:
Enhance laboratory infrastructure (3/15 interviews)
A key opportunity for genetic service delivery identified by clinician participants was to create local capacity to administer the tests, halting the very costly process of internationally outsourcing all testing (Table 5: 4). Maintenance and swift repair of laboratory equipment were also identified as a key factor for sustained genetic service delivery (Table 5: 4).
Discussion
The recent focus on building capacity in genomics in Africa holds promise to transform and modernize the standard of care for genetic conditions, but only if these capacity improvements are linked to clinical care (H3Africa Consortium 2014; Nordling 2017). This will require the strengthening of clinical genetic services. Our study examined HP perceptions of the opportunities and barriers for genetic service delivery in Kenya. Participants included both physicians (who performed genetic counselling) and laboratory directors (who provided genetic testing services), ensuring that perspectives were obtained from different sides of the genetic services sector (Table 1). Our study yielded no participants who reported being certified medical geneticists or genetic counsellors, suggesting that these roles are rare or non-existent in Kenya. There is currently no professional board or council governing genetic counsellors in Kenya (Ormond et al. 2018).
Participants generally perceived that genetics education was limited in the medical school curriculum and revealed that they themselves held limited knowledge about the genetic testing. In the survey, most participants reported obtaining some type of genetics education (Table 1). It was through the interviews, however, that participants further explained that this education did not necessarily prepare physicians to understand the specifics of genetic tests, nor counsel individuals on their results. Participants also expressed that they had no local resources that they could access to increase their knowledge of genetics. Our own prior work highlighted the gap in genetics training for management of retinoblastoma in Kenya (He et al. 2014; Hill et al. 2015).
Participants offered much discussion on the high cost of genetic services as a significant barrier for their delivery to the public. Economic barriers are known to prevent the use of genetic services (Kieran et al. 2007; Vadaparampil et al. 2011), and participants in our study highlighted the financial burden to patients as a significant challenge. Participants further identified the lack of insurance coverage as a substantial barrier preventing the uptake of genetic services. Some clinicians would not recommend certain tests or procedures because they understood that patients could not afford them. Participants identified that insurance offered by international companies seemed to most comprehensively cover genetic testing, whereas local insurance did not offer adequate coverage. Studies have shown that physician recommendation of services have a significant effect on their uptake by the public; therefore, finding ways to lower the cost of genetic services could be a key precursor to their increased use in Kenya (Delikurt et al. 2015; Koil et al. 2003; Vadaparampil et al. 2011). The National Health Insurance Fund, the Kenyan government’s social health insurance scheme, could potentially cover genetic testing in future. Health economic assessment could be pivotal in demonstrating that the medical impact is equal to or outweighs the cost incurred by public health funds.
Participants perceived that the largest contributor to cost was the outsourcing of genetic tests internationally and reported that 50% or more of the tests (excluding PCR & RT-PCR) were outsourced internationally (Table 3). Indeed, the weeks to months that pass before results are returned, and the added cost associated with shipping samples internationally, have been shown to have a negative impact on patients (Delikurt et al. 2015; Kaden and Feinberg 2006; Koil et al. 2003). However, it could arguably be more costly to develop local genetic services if they are not met with sufficient demand. To this point, participants insisted that there was an opportunity for the local development of genetic services due to a steady increase of the public’s awareness of them. Prior studies have shown that both healthcare professional and patient awareness of the services offered are a key factor in their uptake (Beene-Harris et al. 2007; Koil et al. 2003). As exposure and awareness increases, it will serve to further drive demand for genetic services, providing ample opportunity for local laboratories to develop cost-effective services. However, accessibility and availability of medicines for non-communicable diseases is still a challenge for many Kenyans (Onyango et al. 2018). As a result, many patients navigate complex barriers and pay out-of-pocket for drugs, adding financial strain on many households. While increasing the ability to diagnose genetic conditions and prescribe medication is helpful, it should be considered within the broader Kenyan healthcare setting.
Genetic counselling services do not exist in Kenya for many diseases. When they are available, such as the case of retinoblastoma, physicians often provide rudimentary genetic counselling based on disease phenotype (He et al. 2014). Accordingly, participants reported that the lack of guidelines for genetic services posed a significant barrier to their uptake. While there are some recommendations from the Kenyan Ministry of Medical Services for the use of genetic counselling in the presence and absence of genetic results, participants identified that the lack of infrastructure for genetic services contributed to variability in quality (He et al. 2014; Kenyan Ministry of Health and the Kenyan National Retinoblastoma Strategy Group 2014). This is supported by the descriptive statistics generated by this study, as we observed variation among HPs who offered a form of genetic counselling in terms of dedicated time for counselling, duration of initial counselling and follow up, and the resources given to patients (Table 2). Initiatives such as the Kenya National Retinoblastoma Strategy (KNRbS), which established clinical practice guidelines for the management of retinoblastoma in Kenya, including genetic testing and counselling, provide a roadmap for developing the type of guidelines that are needed across the genetic services sector (He et al. 2014). As the demand for genetic resources increases, health law and clinical guidelines surrounding genetic services will likely become a more pressing issue.
Study participants further identified that a significant barrier to the delivery of genetic services was the difficulty communicating genetic information to patients. With many regional languages spoken in Kenya and the majority of technical genetics terms not having analogous words in languages other than English, translating test results poses a significant hurdle. In addition, participants reported that translating results into non-technical terms was difficult, mainly due to the lack of formal training in genetic counselling. Studies have recommended attempting to simplify the message through the use of metaphors related to the patient’s daily life, using locally relevant examples (Gedleh et al. 2018; He et al. 2014). For example, the concept of inheritance of genetic traits could be described through the use of farming metaphors, such as disease resistance in crops or livestock (Marsh et al. 2010). The effective use of culturally relevant terminology has been shown to increase understanding, improve efficacy, and potentially reduce stigma around genetic conditions (Atkin et al. 2009; Tekola et al. 2009). Culturally appropriate terminology may also address another participant reported communication barrier: inexperience dealing with the stigma surrounding genetic conditions. Participants spoke about genetic diagnoses irreparably damaging the relationships between mothers and fathers due to one family member accepting “blame” for the condition, as well as the social isolation those diagnosed with a genetic condition may face in the community due to traditional beliefs surrounding their diagnosis. Studies have suggested that genetic testing and counselling services hold the potential to improve understanding among patient families and possibly prevent family conflict and social stigma (Gedleh et al. 2018). Genetic counsellors would be ideal candidates to provide these services, but in their absence (due to lack of local training programs and employment), alternative models incorporating disease specialists or community healthcare workers with specific knowledge about a condition and ideas on how to more effectively communicate the relevant medical facts could be effective. Indeed, participants reported that a significant opportunity for genetic service delivery was the formation of interdisciplinary teams, fostering better communication between genetic testers and physicians acting as genetic counsellors to improve patient care. This multidisciplinary model has seen success in different parts of Africa: A multidisciplinary team of gynecologist-obstetricians, psychologists, and geneticists in Cameroon work together to determine the most effective way of counselling, and in South Africa, their genetic counselling program incorporated experiences of a medical social worker (Kromberg et al. 2013; Nguefack et al. 2012). It is not unreasonable to suggest that a similar model could be incorporated into the Kenyan healthcare system and see similar levels of success.
While this study is focused on the opportunities and challenges of genetic service delivery from the perspective of Kenyan HPs, further research, partnerships, and engagement are needed in order to create a model that adequately addresses pressing healthcare, cultural, and socioeconomic issues in the Kenyan context.
Genetic services in a Post-COVID-19 Kenya
As the COVID-19 pandemic continues to unfold in Kenya and across the African continent, efforts are being made to contain the effect of the virus by instituting physical distancing measures and economic stimulus packages. However, whole genome sequencing, which is crucial for contact tracing and identifying mutations and strains, is also an important measure that has been garnering significantly less attention, especially in East Africa. As of the time of writing, there are currently no SARS-CoV-2 genomes from Kenya that have been shared with the international database (Ateka et al. 2020; Jerving Sara 2020).
While focusing attention and funds to build genetic service capacity in Kenya may seem discretionary at this time, it could lead to opportunities beyond the ones outlined by our study. For example, 54gene, a Nigerian private genomic start-up established in January 2019 to uncover the genomic basis of non-communicable diseases in Africa (Molteni 2019), has pivoted to helping the Nigerian government provide screening tests for the SARS-CoV-2. With their established scientific and technical capacity centered on molecular diagnostics, 54gene has now set up mobile laboratories in three Nigerian states (NM Partners 2020). This is an example of a made-in-Africa success story showing that investment in a sustainable and context-specific model for genetic service delivery can build capacity for tackling subsequent unforeseen health challenges and prevent further disease burden.
Study limitations
The main limitation of this study was the limited number of participants. While only 15 interviews were conducted in total, this small number is likely directly correlated to the limited number of individuals offering genetic services in Kenya. A combination of busy schedules and limited availability of some HPs meant that additional barriers and opportunities may have been missed. In addition, as researchers were based out of Nairobi while conducting interviews, the perspectives of HPs who worked in other major cities (i.e., Mombasa and Eldoret) may have been missed. That being said, it is likely that most genetic services are available in Nairobi, and themes uncovered by our study were consistently expressed across the conducted interviews and can be seen in analogous contexts in the literature. The fact that we reached saturation of themes with the 15 participants indicates that the addition of more participants would have been unlikely to yield further insight. Another limitation is that some of the participating sites performed testing that may have been outside a medical scope (e.g., DNA paternity testing for social/legal reasons), potentially introducing bias into our data collection and analysis. However, we do not expect this to have been a major source of bias, as the semi-structured interview guide was designed to elicit responses related to testing and counselling for genetic disorders.
Conclusion
The study explored the perspectives of Kenyan HPs who offer genetic services to gain context-specific information about the barriers and opportunities for genetic service delivery. These findings are essential for the development of an accessible, evidence-based, culturally appropriate, and collaborative model of genetic service delivery in Kenya.
Data availability
Available upon request.
Code availability
Not applicable.
References
Ateka E, Ndunguru J, Maeda D, Sseruwagi P, Boykin L (2020) Where are the SARS-CoV-2 genomes from East Africa? Biotechniques. https://www.biotechniques.com/coronavirus-news/opinion_where-are-the-sars-cov-2-genomes-from-east-africa/. Accessed 26 Aug 2020
Atkin K, Ali N, Chu C (2009) The politics of difference? Providing a cancer genetics service in a culturally and linguistically diverse society. Diversity in Health and Care 6(3):149–157
Beene-Harris RY, Wang C, Bach JV (2007) Barriers to access: results from focus groups to identify genetic service needs in the community. Public Health Genomics 10(1):10–18
Burke W (2002) Genetic testing. N Engl J Med 347(23):1867–1875
Canadian Association of Genetic Counsellors (2021) What is a genetic counsellor? https://www.cagc-accg.ca/index.php?page=319. Accessed 21 Mar 2020
Delikurt T, Williamson GR, Anastasiadou V, Skirton H (2015) A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet 23(6):739
Gedleh A, Lee S, Hill JA, Umukunda Y, Qaiser S, Kabiru D, H. (2018) “Where does it come from?” Experiences among survivors and parents of children with retinoblastoma in Kenya. J Genet Couns 27(3):574–588
Genetics Home Reference (2019) What is a genetic consultation? https://ghr.nlm.nih.gov/primer/consult/consultation. Accessed 26 Aug 2020
H3Africa Consortium (2014) Enabling the genomic revolution in Africa. Science 344(6190):1346–1348
Hawkins AK, Hayden MR (2011) A grand challenge: providing benefits of clinical genetics to those in need. Genet Med 13(3):197
He LQ, Njambi L, Nyamori JM, Nyenze EM, Kimani K, Matende I ... Gachago M (2014) Developing clinical cancer genetics services in resource-limited countries: the case of retinoblastoma in Kenya. Public Health Genomics 17(4): 221–227
Hill JA, Lee SY, Njambi L, Corson TW, Dimaras H (2015) Cancer genetics education in a low-to middle-income country: evaluation of an interactive workshop for clinicians in Kenya. PLoS ONE 10(6):e0129852
Jerving Sara (2020) Strengthening Africa’s ability to ‘decode’ the coronavirus. Devex International Development. https://www.devex.com/news/strengthening-africa-s-ability-to-decode-the-coronavirus-97319. Accessed 26 Aug 2020
Kaden PA, Feinberg B (2006) Pros and cons of outsourcing laboratory services. J Oncol Pract 2(4):162–163
Kenyan Ministry of Health and the Kenyan National Retinoblastoma Strategy Group (2014) Retinoblastoma Best Practice Guidelines. Available at: http://guidelines.health.go.ke:8000/media/Best-Practice-Retinoblastoma_September-2014.pdf. Accessed 18 May 2021
Kieran S, Loescher LJ, Lim KH (2007) The role of financial factors in acceptance of clinical BRCA genetic testing. Genet Test 11(1):101–110
Koil CE, Everett JN, Hoechstetter L, Ricer RE, Huelsman KM (2003) Differences in physician referral practices and attitudes regarding hereditary breast cancer by clinical practice location. Genet Med 5(5):364
Kromberg JG, Sizer EB, Christianson AL (2013) Genetic services and testing in South Africa. J Community Genet 4(3):413–423
Lee S, Gedleh A, Hill JA, Qaiser S, Umukunda Y, Odiyo P … Dimaras H (2017) In their own words: a qualitative study of Kenyan breast cancer survivors’ knowledge, experiences, and attitudes regarding breast cancer genetics. J Glob Oncol 4: 1–9
Marsh VM, Kamuya DM, Mlamba AM, Williams TN, Molyneux SS (2010) Experiences with community engagement and informed consent in a genetic cohort study of severe childhood diseases in Kenya. BMC Med Ethics 11(1):13
Marsh V, Kombe F, Fitzpatrick R, Williams TN, Parker M, Molyneux S (2013) Consulting communities on feedback of genetic findings in international health research: sharing sickle cell disease and carrier information in coastal Kenya. BMC Med Ethics 14:41. https://doi.org/10.1186/1472-6939-14-41
McPherson E (2006) Genetic diagnosis and testing in clinical practice. Clin Med Res 4(2):123–129
Molteni M (2019) The massive, overlooked potential of African DNA. Wired. https://www.wired.com/story/the-massive-overlooked-potential-of-african-dna/. Accessed 26 Aug 2020
Nguefack CT, Brulet C, Njiengwe E, Sandjon G, Onomo M, Kamgaing JT, … Doualla C (2012) Douala prenatal diagnosis staff (Cameroon): four years of activity. Prenat Diagn 32(1): 94–96.
NM Partners (2020) Aliko Dangote Foundation engages 54gene laboratory to conduct 1,000 COVID-19 tests per day in Kano. Nairametrics. https://nairametrics.com/2020/05/04/aliko-dangote-foundation-engages-54gene-laboratory-to-conduct-1000-covid-19-tests-per-day-in-kano/. Accessed 26 Aug 2020
Nordling L (2017) How the genomics revolution could finally help Africa. Nature News 544(7648):20
Onyango MA, Vian T, Hirsch I, Salvi DD, Laing R, Rockers PC, Ashigbie PG, Wirtz VJ (2018) Perceptions of Kenyan adults on access to medicines for noncommunicable diseases: A qualitative study. PLoS One 13(8):e0201917. https://doi.org/10.1371/journal.pone.0201917
Ormond KE, Laurino MY, Barlow-Stewart K, Wessels TM, Macaulay S, Austin J, Middleton A (2018) Genetic counseling globally: where are we now? Am J Med Genet C Semin Med Genet 178(1):98–107
Resta R, Biesecker BB, Bennett RL, Blum S, Hahn SE, Strecker MN, Williams JL (2006) A new definition of genetic counseling: National Society of Genetic Counselors’ task force report. J Genet Couns 15(2):77–83
Tekola F, Bull S, Farsides B, Newport MJ, Adeyemo A, Rotimi CN, Davey G (2009) Impact of social stigma on the process of obtaining informed consent for genetic research on podoconiosis: A qualitative study. BMC Med Ethics 10(1):13
Trepanier AM, Allain DC (2014) Models of service delivery for cancer genetic risk assessment and counseling. J Genet Couns 23(2):239–253
Vadaparampil ST, Quinn GP, Dutil J, Puig M, Malo TL, McIntyre J, … Closser Z (2011) A pilot study of knowledge and interest of genetic counseling and testing for hereditary breast and ovarian cancer syndrome among Puerto Rican women. J Community Genet 2(4): 211–221
Verma IC, Puri RD (2015) Global burden of genetic disease and the role of genetic screening. Semin Fetal Neonatal Med 20(5):354–63. https://doi.org/10.1016/j.siny.2015.07.002
Wonkam A, Tekendo CN, Sama DJ, Zambo H, Dahoun S, Bena F, Morris MA (2011) Initiation of a medical genetics service in Sub-Saharan Africa: experience of prenatal diagnosis in Cameroon. Eur J Med Genet 54(4):e399–e404
World Health Organization (2005) Control of genetic diseases. Report. Available from: http://apps.who.int/gb/archive/pdf_files/EB116/B116_3-en.pdf. Accessed 18 May 2021
Zhong A, Darren B, Loiseau B, He LQB, Chang T, Hill J, Dimaras H (2018) Ethical, social, and cultural issues related to clinical genetic testing and counseling in low- and middle-income countries: a systematic review. Genet Med. https://doi.org/10.1038/s41436-018-0090-9
Acknowledgements
We thank Sangeetha Paramathas for the critical review of the manuscript.
Funding
Authors AZ, KX and ZH were supported by the Queen Elizabeth II Diamond Jubilee Trust Scholars program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in this study involving human participants were in accordance with the ethical standards of the University of Nairobi, the University of Toronto, and with the Declaration of Helsinki of 1975, as revised in 2000 (5). Written informed consent was obtained from all parties prior to their participation in the study interview.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhong, A., Xia, K., Hadjis, Z. et al. Opportunities and barriers for genetic service delivery in Kenya from a health personnel perspective. J Community Genet 12, 525–538 (2021). https://doi.org/10.1007/s12687-021-00532-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12687-021-00532-5