Abstract
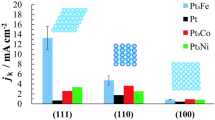
Pt3Fe(111), Pt3Fe(775) = 7(111)–(111), and Pt3Fe(544) = 9(111)–(100) electrodes show the highest activity in the low-index planes, n(111)–(111), and n(111)–(100) series of Pt3Fe, respectively. The surfaces of these electrodes were modified with hydrophobic species such as THA+, melamine, and ionic liquid ([MTBD][beti]), and the effects on the oxygen reduction reaction (ORR) were studied. All the hydrophobic species improved the ORR activity on all the electrodes examined. The ORR activity of Pt3Fe(111) in 0.1 M HClO4 containing 0.1 μM melamine was 2.1 times higher than that of Pt3Fe(111) without melamine, giving 39 times higher activity than that of bare Pt(111). The durability was improved on all the electrodes examined in melamine-containing solution.










Similar content being viewed by others
Data Availability
Supporting data are available.
References
N.M. Marković, R.R. Adzić, B.D. Cahan, E.B. Yeager, Structural effects in electrocatalysis: Oxygen reduction on platinum low index single–crystal surfaces in perchloric acid solutions. J. Electroanal. Chem. 377, 249–259 (1994). https://doi.org/10.1016/0022-0728(94)03467-2
M.D. Maciá, J.M. Campiña, E. Herrero, J.M. Feliu, On the kinetics of oxygen reduction on platinum stepped surfaces in acidic media. J. Electroanal. Chem. 564, 141–150 (2004). https://doi.org/10.1016/j.jelechem.2003.09.035
A. Kuzume, E. Herrero, J.M. Feliu, Oxygen reduction on stepped platinum surfaces in acidic media. J. Electroanal. Chem. 599, 333–343 (2007). https://doi.org/10.1016/j.jelechem.2006.05.006
A. Hitotsuyanagi, M. Nakamura, N. Hoshi, Structural effects on the activity for the oxygen reduction reaction on n(111)–(100) series of Pt: Correlation with the oxide film formation. Electrochim. Acta 82, 512–516 (2012). https://doi.org/10.1016/j.electacta.2012.03.133
N. Hoshi, M. Nakamura, A. Hitotsuyanagi, Active sites for the oxygen reduction reaction on the high–index planes of Pt. Electrochim. Acta 112, 899–904 (2013). https://doi.org/10.1016/j.electacta.2013.05.045
Y. Takesue, M. Nakamura, N. Hoshi, Structural effects on the oxygen reduction reaction on the high index planes of Pt3Co. Phys. Chem. Chem. Phys. 16, 13774–13779 (2014). https://doi.org/10.1039/C4CP00243A
T. Rurigaki, A. Hitotsuyanagi, M. Nakamura, N. Sakai, N. Hoshi, Structural effects on the oxygen reduction reaction on the high index planes of Pt3Ni: n(111)–(111) and n(111)–(100) surfaces. J. Electroanal. Chem. 716, 58–62 (2014). https://doi.org/10.1016/j.jelechem.2013.12.008
V.R. Stamenkovic, B. Fowler, B.S. Mun, G. Wang, P.N. Ross, C.A. Lucas, N.M. Markovic, Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science. 315, 493–497 (2007). https://doi.org/10.1126/science.1135941
R. Jinnouchi, K. Kodama, Y. Moromoto, DFT calculations on H, OH and O adsorbate formations on Pt(111) and Pt(332) electrodes. J. Electroanal. Chem. 716, 31–44 (2014). https://doi.org/10.1016/j.jelechem.2013.09.031
T. Toda, H. Igarashi, H. Uchida, M. Watanabe, Enhancement of the electroreduction of oxygen on Pt alloys with Fe, Ni, and Co. J. Electrochem. Soc. 146, 3750–3756 (1999). https://doi.org/10.1149/1.1392544
V. Stamenkovic, T.J. Schmidt, P.N. Ross, N.M. Markovic, Surface composition effects in electrocatalysis: Kinetics of oxygen reduction on well–defined Pt3Ni and Pt3Co alloy surfaces. J. Phys. Chem. B 106, 11970–11979 (2002). https://doi.org/10.1021/jp021182h
B. Hammer, J.K. Nørskov, Theoretical surface science and catalysis–calculations and concepts. Adv. Catal. 45, 71–129 (2000). https://doi.org/10.1016/S0360-0564(02)45013-4
A. Kulkarni, S. Siahrostami, A. Patel, J.K. Nørskov, Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018). https://doi.org/10.1021/acs.chemrev.7b00488
V. Stamenkovic, B.S. Mun, K.J. Mayrhofer, P.N. Ross, N.M. Markovic, J. Rossmeisl, J. Greeley, J.K. Nørskov, Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. 118, 2963–2967 (2006). https://doi.org/10.1002/anie.200504386
M. Wakisaka, S. Kobayashi, S. Morishima, Y. Hyuga, D.A. Tryk, M. Watanabe, A. Iiyama, H. Uchida, Unprecedented dependence of the oxygen reduction activity on Co content at Pt skin/Pt–Co(111) single crystal electrodes. Electrochem. Commun. 67, 47–50 (2016). https://doi.org/10.1016/j.elecom.2016.03.015
S. Kobayashi, M. Wakisaka, D.A. Tryk, A. Iiyama, H, Uchida, Effect of alloy composition and crystal face of Pt–skin/Pt100–xCox [(111), (100), and (110)] single crystal electrodes on the oxygen reduction reaction activity. ACS Omega 121, 11234–11240 (2017). https://doi.org/10.1021/acs.jpcc.6b12567
S. Kobayashi, M. Aoki, M. Wakisaka, T. Kawamoto, R. Shirasaka, K. Suda, D.A. Tryk, J. Inukai, T. Kondo, H. Uchida, Atomically flat Pt skin and striking enrichment of Co in underlying alloy at Pt3Co(111) single crystal with unprecedented activity for the oxygen reduction reaction. ACS Omega 3, 154–158 (2018). https://doi.org/10.1021/acsomega.7b01793
C. Wang, D. Vilet, K.L. More, N.J. Zaluzec, S. Peng, S. Sun, H. Daimon, G. Wang, J. Greeley, J. Pearson, A.P. Paulikes, G. Karaprtrov, D. Strmcnik, N.M. Markovic, V.R. Stamenkovic, Multimetallic Au/FePt3 nanoparticles as highly durable electrocatalyst. Nano Lett. 11, 919–926 (2011). https://doi.org/10.1021/nl102369k
Y. Shi, W. Yang, W. Gong, X. Wang, Y. Zhou, X. Shen, Y. Wu, J. Di, D. Zhang, Q. Li, Interconnected surface–vacancy–rich PtFe nanowires for efficient oxygen reduction. J. Mater. Chem. A 9, 12845–12852 (2021). https://doi.org/10.1039/D1TA00972A
A. Suzuki, M. Nakamura, N, Hoshi, Effects of surface structures and hydrophobic species on the oxygen reduction reaction activity of Pt3Fe single–crystal electrodes. Electrocatalysis 13, 175–181 (2022). https://doi.org/10.1007/s12678-021-00699-y
A. Suzuki, M. Nakamura, N, Hoshi, Structural effects of the oxygen reduction reaction on the high index planes of Pt3Fe. Electrochem Commun. 136, 107235–107241 (2022). https://doi.org/10.1016/j.elecom.2022.107235
J.X. Wang, N.M. Markovic, R.R. Adzic, Kinetic analysis of oxygen reduction on Pt(111) in acid solutions: Intrinsic kinetic parameters and anion adsorption effects. J. Phys. Chem. B 108, 4127–4133 (2004). https://doi.org/10.1021/jp037593v
H. Tanaka, S. Sugawara, K. Shinohara, T. Ueno, S. Suzuki, N. Hoshi, M. Nakamura, Infrared reflection absorption spectroscopy of OH adsorption on the low index planes of Pt. Electrocatalysis 6, 295–299 (2015). https://doi.org/10.1007/s12678-014-0245-7
T. Ueno, H. Tanaka, S. Sugawara, K. Shinohara, A. Ohma, N. Hoshi, M. Nakamura, Infrared spectroscopy of adsorbed OH on n(111)-(100) and n(111)-(111) series of Pt electrode. J. Electroanal. Chem. 800, 162–166 (2017). https://doi.org/10.1016/j.jelechem.2016.11.028
K. Miyabayashi, H. Nishihara, M. Miyake, Platinum nanoparticles modified with alkylamine derivatives as an active and stable catalyst for oxygen reduction reaction. Langmuir 30, 2936–2942 (2014). https://doi.org/10.1021/la402412k
K. Saikawa, M. Nakamura, N. Hoshi, Structural effects on the enhancement of ORR activity on Pt single–crystal electrodes modified with alkylamines. Electrochem. Commun. 87, 5–8 (2018). https://doi.org/10.1016/j.elecom.2017.12.016
N. Hoshi, K. Saikawa, M. Nakamura, Structural effects on water molecules on the low index planes of Pt modified with alkyl amines and the correlation with the activity of the oxygen reduction reaction. Electrochem. Cummun. 106, 106536–106541 (2019). https://doi.org/10.1016/j.elecom.2019.106536
M. Asahi, S. Yamazaki, N. Taguchi, T. Ioroi, Facile approach to enhance oxygen reduction activity by modification of platinum nanoparticles by melamine–formaldehyde polymer. J. Electroanal. Soc. 166, 498–506 (2019). https://doi.org/10.1149/2.0641908jes
N. Wada, M. Nakamura, N. Hoshi, Structural effects on the oxygen reduction reaction on Pt single–crystal electrodes modified with melamine. Electrocatalysis 11, 275–281 (2020). https://doi.org/10.1007/s12678-020-00584-0
T. Kumeda, H. Tajiri, O. Sakata, N. Hoshi, M. Nakamura, Effect of hydrophobic cations on the oxygen reduction reaction on single–crystal platinum electrodes. Nat. Commun. 9, 4378–4384 (2018). https://doi.org/10.1038/s41467-018-06917-4
Y. Li, J. Hart, L. Profitt, S. Intikhab, S. Chatterjee, M. Taheri, J. Snyder, Sequential capacitive deposition of ionic liquids for conformal thin film coatings on oxygen reduction reaction electrocatalysts. ACS Catal. 9, 9311–9316 (2019). https://doi.org/10.1021/acscatal.9b03157
J. Clavilier, R. Faure, G. Guinet, R. Durand, Preparation of monocrystalline Pt microelectrodes and electrochemical study of the plane surfaces cut in the direction of the (111) and (110) planes. J. Electroanal. Chem. 107, 205–209 (1980). https://doi.org/10.1016/S0022-0728(79)80022-4
B.D. Cahan, H.M. Villullas, The hanging meniscus rotating disk (HMRD). J. Elecroanal. Chem. 307, 263–268 (1991). https://doi.org/10.1016/0022-0728(91)85553-2
H.M. Villullas, M.L. Teijelo, The hanging meniscus rotating disk (HMRD) Part 1. Dependence of hydrodynamic behavior on experimental variables. J. Electroanal. Chem. 384, 25–30 (1995).
H.M. Villullas, M.L. Teijelo, The hanging meniscus rotating disk (HMRD) Part 2. Application to simple charge transfer reaction kinetics. J. Electroanal. Chem. 385, 39–44 (1995).
J. Clavilier, K. El Achi, A. Rodes, In situ probing of step and terrace sites on Pt(S)–[n(111) × (111)] electrodes. Chem. Phys. 141, 1–14 (1990). https://doi.org/10.1016/0301-0104(90)80014-O
A. Rodes, K. El Achi, M.A. Zamakhchari, J. Clavilier, Hydrogen probing of step and terrace sites on Pt(S)–[n(111) × (100)]. J. Electroanal. Chem. 284, 245–253 (1990). https://doi.org/10.1016/0022-0728(90)87077-W
N.M. Markovic, H.A. Gasteiger, P.N. Ross Jr., Oxygen reduction on platinum low–index single–crystal surfaces in sulfuric acid solution: Rotating ring–Pt(hkl) disk studies. J. Phys. Chem. 99, 3411–3415 (1995). https://doi.org/10.1021/j100011a001
Funding
This study was partially supported by New Energy and Industrial Technology Development Organization (NEDO) 20001187-0.
Author information
Authors and Affiliations
Contributions
Akane Suzuki did investigation, wrote the main manuscript text, prepared Figs. 1, 2, 3, 4, 5, 6, 7, 8, and 9. Masashi Nakamura supervised, reviewed and edited the manuscript. Haruki Shimada did DFT calculation, prepared Fig. 10. Nagahiro Hoshi conceptualized, supervised, administrated the project, acquired funding, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethical Approval and Consent to Participate
All authors approve human ethics and consent to participate.
Consent for Publication
All authors consent to publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suzuki, A., Nakamura, M., Shimada, H. et al. Effects of Hydrophobic Species on the Oxygen Reduction Reaction on the High-Index Planes of Pt3Fe. Electrocatalysis 14, 306–314 (2023). https://doi.org/10.1007/s12678-022-00795-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-022-00795-7