Abstract
Purpose
This study aimed to elucidate the predictive role of an oxidative stress-related genes (OSRGs) model in colon cancer.
Materials and methods
First, OSRGs that were differentially expressed between tumor and normal tissues were identified using The Cancer Genome Atlas (TCGA)—(Colorectal Adenocarcinoma) COAD dataset. Then, Lasso COX regression was performed to develop an optimal prognostic model patients were stratified into high- and low-risk groups based on the expression patterns of these genes. The model's validity was confirmed through Kaplan–Meier survival curves and receiver operating characteristic curve (ROC) analysis. Additionally, enrichment analyses were performed using Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Gene Set Enrichment Analysis (GSEA) to uncover underlying mechanisms.
Results
A totally of 115 differentially expressed OSRGs were identified within the TCGA cohort, with 17 significantly linked to overall survival. These 17 genes were used to formulate a prognostic model that differentiated patients into distinct risk groups, with the high-risk group demonstrating a notably inferior overall survival rate. The risk score, when integrated with clinical and pathological data, emerged as an independent prognostic indicator of colon cancer. Further analyses revealed that the disparity in prognostic outcomes between risk groups could be attributed to the reactive oxygen species pathway and the p53 signaling pathway.
Conclusion
A new prediction model was established based on OSRGs. CYP19A1, NOL3 and UCN were found to be highly expressed in tumor tissues and substantial clinical predictive significance. These findings offer new insights into the role of oxidative stress in colon cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
At present, colorectal cancer (CRC) is third most prevalent malignancy and a significant cause of cancer-related mortality in the United States, with an estimated five-year survival rate of 65% [1, 2]. Although its initial presentation is often insidious and screening techniques are varied, including endoscopy, stool examination, blood tests, imaging, CRC is often characterized by rapid progression [3, 4]. As a result, a substantial proportion of patients are diagnosed at advanced stages, when the optimal window for therapeutic intervention has already passed. Furthermore, the post-treatment prognosis of CRC patients is marred by the persistent challenges of tumor metastasis and recurrence, which contribute to the suboptimal long-term survival rates [5].
Currently, clinical decision-making for CRC patients predominantly relies on a spectrum of clinical and pathological tumor characteristics, including but not limited to gender, age, TNM (Tumor Node Metastasis) staging, and tumor marker profiles. However, given the pronounced clinical heterogeneity inherent to CRC, these conventional prognosticators fall short in accurately forecasting individual patient outcomes [6]. Concurrently, the advent of gene expression-based predictive models has contributed to significant progress in the assessment of CRC prognosis, exemplified by models that account for fibroblast activity [7], immune-related risk models [8], and the pyroptosis-related models [9].
Tumor microenvironment (TME) oxidative stress mainly originates from tumor epithelial cells and is mainly caused by the accumulation of reactive oxygen species (ROS) in tumor cells [10,11,12]. A low ROS concentration can promote tumor glucose metabolism and maintain the high energy consumption requirements of tumor. A high concentration of ROS can induce apoptosis of tumor cells and inhibit the occurrence and development of tumors [13]. Furthermore, evidence suggests that oxidative stress has plays a role in triggering metabolic and genomic alterations that could potentially accelerate tumor metabolism by modulating the activity of key metabolic enzymes and signaling pathways, thus promoting tumor development [14]. When oxidative stress occurs, many cellular biomolecules, such as deoxyribonucleic acid, lipids and proteins, can cause dysfunction of cells, tissues, and organs due to oxidative damage caused by free radicals [15]. The response of HIF and NRF2 signaling pathways to hypoxia and oxidative stress has become an important target for cancer treatment [16]. Targeting specific genes or pathways that promote oxidative stress-induced cellular senescence during the stage of cancer progression can inhibit cancer progression [17]. Thus, oxidative stress plays an important role in tumor stress, however, the role of oxidative stress-related genes in the prognosis of colon cancer has not been studied.
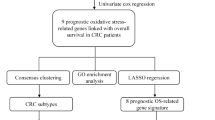
In this study, we explored the expression of oxidative stress-related genes (OSRGs) in colon cancer (CC) patients using data from a public database—The Cancer Genome Atlas (TCGA). Further, a model of OSRGs closely related to CC prognosis was established, severing as an independent prognostic indicator of CC. Subsequent functional enrichment analysis was performed to reveal the pathways associated with cancer development.
2 Materials and methods
2.1 Human oxidative stress-related genes (OSRGs) dataset
The Genecards database (https://www.genecards.org) was searched for genes with relevance scores greater than 7 that are involved in oxidative stress. A total of 1094 genes were identified, as shown in the supplement.
2.2 Samples and data collected for this study
Clinical data and ribonucleic acid sequencing (RNA-seq) expression data of CC patients were downloaded from the UCSC XENA website (https://xenabrowser.net/datapages). After excluding patients with incomplete clinical data and a follow-up period of less than 30 days, the clinical data and RNA sequencing expression data were obtained. Because this was a retrospective analysis based on a public database, ethical approval was not required.
2.3 Differential expression analysis
Differentially expressed genes (DEGs) was identified using count data based on input of the OSRGs input to the R package “DESeq2” [18]. Specifically, DEGs were defined as those with an absolute log2 (fold-change) value greater than 2 and a significant p value (p < 0.05).
2.4 Univariate COX regression analysis
The “Survival” and “Survminer” R packages were employed to identify OSRGs with prognostic significance. From the initial Genecards database of 1094 genes, a refined subset of 115 prognostic OSRGs was discerned through univariate Cox regression analysis, predicated on their gene expression profiles.
2.5 Construction of ORGs-related prognostic model
Seventeen prognostic genes were identified using Lasso COX regression analysis[19] based on the 115 prognostic OSRGs. After the expression values of each prognostic gene were combined, a risk score formula for each patient was constructed and its estimated regression coefficients were weighted by Lasso COX regression analysis (Table 1). The risk score was calculated as follows: risk score = \({\sum }_{i=1}^{n}{expr}_{i}{\beta }_{i}\), where expr represents the prognostic gene expression value and β represents the Lasso coefficient.
The formula was used to score the risk of each patient in the TCGA cohort, and the patients were divided into a low-risk group and a high-risk group with the median risk score as the cut-off point. The Kaplan–Meier method was used to evaluate the difference in survival between the two groups; a between-groups comparison was performed using the log-rank statistical method. The predictive efficacy of the risk score in forecasting patient prognosis was further scrutinized through a Cox regression analysis, providing a robust statistical framework to evaluate the prognostic value of the model.
2.6 Functional enrichment analysis and genomic interaction analysis
To unravel the biological underpinnings of the prognostic genes, a comprehensive suite of functional enrichment analyses was performed. This included Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and Gene Set Enrichment Analysis (GSEA), aimed at identifying pivotal biological pathways and processes.
2.7 Immune infiltration analysis
Using the CIBERSORT deconvolution algorithm, the cellular composition was estimated based on the gene expression profiles, thereby providing quantification of relative abundance of 22 distinct immune cell types within each sample [20, 21].
2.8 Statistical analysis
The Cox proportional risk model was used to analyze univariate and multivariate survival. All statistical analyses were performed in R. All statistical tests were bilateral. p < 0.05 was considered statistically significant.
3 Results
3.1 Identification of differentially expressed OSRGs
RNA-seq expression data from 453 tumor samples and 41 normal samples in the TCGA-COAD dataset were used to identify DEGs. The expression values of 807 OSRGs were extracted. Compared with normal samples, 55 downregulated genes and 32 upregulated genes were identified based on the following criteria: p < 0.05 and [log2 (fold change)] > 2. Among these, the top five genes that were highly expressed in tumor tissue compared to normal tissue are “GDF15”, “MMP3”, “TG”, “TIMP1” and “MMP1”, while the top five genes that were under expressed in tumor samples compared to normal samples were “SGK1”, “PTGS1”, “NR3C2”, “ACADS” and “MYLK”. Scatter plots were constructed to show the expression level of these differentially expressed OSRGs between tumor and normal tissues, as shown in Fig. 1a. A volcano map of the differentially expressed OSRGs is shown in Fig. 1b.
3.2 Construction of a model to predict the prognosis of colon cancer patients in the TCGA cohort using the 17 prognostic OSRGs
A total of 17 OSRGs were significantly correlated with prognosis after Lasso COX regression analysis (Fig. 2a, b). The full names of these 17 genes and their coefficients in the formula are shown in Table 1.
Oxidative stress-related genes model in The Cancer Genome Atlas (TCGA) cohort. a, b Lasso COX analysis of prognostic genes. c The number of patients in the different risk groups. d Survival status of patients in the different groups. e Overall survival (OS) was shorter in the high-risk group compared to the low-risk group f ROC analysis of OS for the model and various clinicopathologic factors
The expression coefficients of 17 risk genes were obtained. The prognostic model based on these 17 genes was established using the following formula: Risk Score = 0.209 × expression level of RYR2) + (-0.229 × expression level of GSR) + (0.049 × expression level of GSTM1) + (0.096 × expression level of HSPA1A) + (0.683 × expression level of ACADL) + (0.080 × expression level of STK25) + (0.423 × expression level of ALOX12) + (0.048 × expression level of MAPK12) + (-0.006 × expression level of SERPINA1) + (0.098 × expression level of CYP19A1) + (0.098 × expression level of NOL3) + (0.033 × expression level of NGF) + (-0.006 × expression level of GDF15) + (0.007 × expression level of NTRK2) + (0.108 × expression level of DAPK1) + (0.032 × expression level of UCN) + (0.024 × expression level of HBA2).
Next, the risk score of each CC patient in the TCGA cohort was calculated and the patients were divided into a high-risk group and a low-risk group using the median risk value as the cut-off point (Fig. 2c, d). Overall survival (OS) was found to be shorter in the high-risk group compared to the low-risk group (Fig. 2e).
A receiver operating characteristic curve (ROC) was used to examine the predictive performance of the model. The AUC value of this model was 0.759, which was significantly higher than the AUC values based on age, gender, TNM stage, T, N and M stages (Fig. 2f). These results suggest that the riskscore characteristic is a better predictor of survival in patients with CC than the other clinical and pathological factors.
The associations between the clinicopathological parameters and the risk model were examined (Fig. 3a–d). The results showed that the risk model was differentially expressed across various clinicopathological parameters, such as TNM stage (p < 0.001), T stage (p < 0.001), N stage (p < 0.001) and M stage(p < 0.001).
Kaplan–Meier curves based on the risk score and the other clinical and pathological factors are shown (Fig. 4a–f). A significant difference in survival was observed between the high-risk and low-risk groups (p < 0.001).
Univariate (Fig. 5a) and multivariate (Fig. 5b) Cox regression analysis were performed to verify the independent predictive ability of the 17-genes model for survival. Univariate Cox analysisfound that risk score (p < 0.001), TNM stage (p < 0.001), T stage (p = 0.003), N stages (p < 0.001) and M stage (p < 0.001) was associated with survival in CC patients. These factors were then included in a multivariate Cox analysis, which confirmed the independent prognostic effects of the risk score characteristics (p < 0.001). A nomogram of the CC predictive model based on the 17-gene riskscore is presented in Fig. 5c.
Univariate (a) and multivariate (b) Cox regression analysis of the survival of colon cancer patients in The Cancer Genome Atlas (TCGA) cohort. c Nomogram of the predictive model based on the 17-gene risk score (Age:1-below 60, 2-over 60; Gender:1-male, 2-female; Stage:1-I, 2-II,3-III, 4-IV; T.Stage:1-T1, 2-T2, 3-T3, 4-T4; N.Stage:1-N0, 2-N1,3-N2; M.Stage:1-M0, 2-M1;Risk:1-Low risk, 2-High risk)
3.3 Kaplan–Meier curves curves based on the 17-genes expression
Firstly, the differential expression of the 17 OSRGs between colon adenocarcinoma tissues and normal tissues was examined. As shown in Fig. 6, STK25, CYP19A1, NOL3, NGF, GDF15, and UCN were upregulated in tumor tissues. GSTM1, HSPA1A, ACADL, DAPK1, MAPK1, and NTRK2 were down-regulated in tumor tissues. In addition, survival curves were calculated and plotted separately for these DEGs between normal and tumor tissues. The significant genes for clinical diagnosis were RYR2, GSR, GSTM1, HSPA1A, ALOX12, SEROINA1, CYP19A1, NOL3, DAPK1, UCN, and HBA2 (Fig. 7). According to these two graphs, the logically correct genes are CYP19A1, NOL3 and UCN; these genes were highly expressed in tumor tissues and have significant clinical predictive significance.
3.4 Functional enrichment based on the 17 genes
Enrichment analysis was conducted based on the risk scores of the 17 OSRGs model, providing a biological understanding of these genes. The GO functional enrichment and the KEGG pathway enrichment analysis of these genes are summarized in Fig. 8a, b. The top enriched GO terms in biological processes were “axon development”, “regulation of membrane”, and “potential synapse organization”, those in cellular components were “neuronal cell body”, “synaptic membrane”, and “collagen-containing extracellular matrix”, and those in molecular function were “channel activity” and “passive transmembrane transporter activity”. In the KEGG pathway enrichment analysis, “neuroactive ligand-receptor interaction”, “adrenergic signaling in cardiomyocytes”, “cell adhesion molecules”, and “calcium signaling pathway” were enriched in the high-risk groups. In the GSEA enrichment results (Fig. 8c), we found that the “adaptive thermogenesis”, “lipid catabolic process”, “regulation of small molecule metabolic process”, “temperature homeostasis” were enriched in the high-risk groups.
3.5 Correlations with immune cells
The association of between the model and the immune cell infiltration in the TME of CC patients was analyzed (Fig. 9a). In Cibersort analysis, the proportions of resting dendritic cells, active dendritic cells, Tregs cells, activated CD4+ T cells and CD4+ memory T cells in the high-risk group was significantly higher than that in the low-risk group. The content of CD8+ T cells did not differ significantly between the two groups (Fig. 9b).
4 Discussion
Oxidative stress plays a pivotal role in carcinogenesis tumor progression [22,23,24,25,26]. The cellular burden of ROS, stemming from both endogenous and exogenous sources, can escalate, leading to the modification and damage of cellular macromolecules, in particular, genomic DNA, thereby seeding the potential for mutations [27, 28]. Despite this, the specific contribution of oxidative stress to the pathogenesis and progression of CC remains an area ripe for deeper investigation. This study is the first to explore the role of oxidative stress in a cohort of colon cancer patients and has prospective significance.
Over the past two decades, the prevalence and burden of colorectal cancer (CRC) have seen a significant rise, disproportionately affecting men and individuals in the middle to older age categories [29]. It is critical that we enhance preventive strategies targeting the principal risk factors at play. Concurrently, the thorough analysis of prognostic indicators has emerged as a vital aspect of our ongoing battle against CRC, essential for improving patient outcomes. Currently, tumor prognosis is predominantly gauged by patient’s clinical and pathological characteristics [30]. The advent of advanced gene sequencing technologies has ushered in a new era where mRNA levels serve as prognostic indicators for an array of cancers. Encouragingly, expansive databases like the TCGA have equipped researchers with robust tools for gene model exploration, facilitating a more profound comprehension of the nexus between OSRGs and CC. In the current study, by leveraging the extant gene expression data from CC patients, we initially pinpointed 115 differentially expressed OSRGs within the TCGA-COAD dataset, marking a significant step towards unraveling the intricate interplay of these genes in CC.
From an oxidative stress-centric perspective, our analysis of CC-related prognostic genes identified 17 significant OSRGs via Lasso Cox regression analyses. These genes were instrumental in the development of a prognostic model capable of stratifying CC patients into distinct risk categories. The application of this 17 OSRGs model to patient data revealed a stark divergence in survival curves between the low- and high-risk groups, with the latter exhibiting markedly reduced survival times. Moreover, patients assigned to the high-risk group consistently presented with more advanced TNM, T, N, and M staging compared to their low-risk counterparts. The independent prognostic significance of our oxidative stress-related model was substantiated by Cox regression analysis, underscoring its efficacy as a metric for gauging the pathogenesis and disease progression, and its critical role in the prognostic assessment of CC patients. We also confirmed that this risk signature was positively correlated with the degree of immune cell infiltration in the CRC tumor microenvironment. In summary, we could predict the survival and immune cell infiltration of CC patients based on this risk signature.
The trio of pivotal genes within our model, CYP19A1, UCN, and NOL3, have each garnered considerable attention in the existing literature. CYP19A1 has been shown to be a promising target for enhancing the efficacy of immunotherapy in colon cancer [31]. UCNs, integral members of the corticotropin-releasing factor (CRF) family, are implicated in a spectrum of biological processes, including inflammation and the orchestration of cancer development [32]. NOL3, encoding an anti-apoptotic protein, is intricately involved in pathways governing apoptosis, autophagy, and signal transduction, with its caspase recruitment domain (CARD) demonstrated to modulate p53 activity through CARD–CARD interactions [33].
GO and KEGG analyses elucidated the involvement of the differentially expressed OSRGs in a risk model predictive of the OS of CC patients. These genes were found to be implicated in multiple biological mechanisms, including the modulation of viral infection and the MAPK signaling pathway, which may contribute to the prognostic heterogeneity observed. GSEA further delineated the divergent molecular landscapes between high-risk and low-risk groups as defined by our oxidative stress-related model. Notably, pathways such as the "reactive oxygen species pathway" and the "p53 signaling pathway" were significantly enriched in the high-risk group, underscoring their potential role in CC prognosis [34, 35].
Immune cell infiltration is associated with CC occurrence and progression. Immunotherapy has considerably extended the period that patients with advanced tumor survive [36]. In our study risk score was significantly and positively correlated with the richness of specific types of immune cell infiltration in the tumor microenvironment. We found that CD4+ memory T cells, dendritic cells, were positively correlated with the OSRGs risk score. Recent breakthroughs in single-cell and spatial transcriptomics have marked a pivotal phase in biological research [37,38,39], which is more helpful to understand the composition and function of immune cells in the tumor microenvironment and the interactions between various immune cell types.
Despite its strengths, there are some limitations of this study that should be noted. First, the data analysis and results validation of this study were conducted using data from public databases, and performance of model was not tested in prospective experiments. Secondly, our study is only retrospective study which needs more prospective clinical trials to verify the efficacy of its predictions. In addition, the mechanism underlying the roles of the 17 OSRG in the occurrence and development of CC still require further studied.
5 Conclusion
In summary, we established a new prediction model of oxidative stress-related genes, which is also helpful for clinicians to make preliminary judgment on patient prognosis. According to above we found CYP19A1, NOL3 and UCN are highly expressed in tumor tissues and have significant clinical predictive significance. Our study provides new insights into the role of oxidative stress in colon cancer.
Data availability
All data in this study came from public database of TCGA. The results of this study are based on the data from TCGA (https://xenabrowser.net/).
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: A Cancer J Clin. 2021;71(1):7–33.
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53.
Yang Y, Gao Z, Huang A, Shi J, Sun Z, Hong H, Gu J. Epidemiology and early screening strategies for colorectal cancer in China. Chin J Cancer Res. 2023;35(6):606–17.
Huang Y, He L, Li Z, Chen X, Han C, Zhao K, Zhang Y, Qu J, Mao Y, Liang C, et al. Coupling radiomics analysis of CT image with diversification of tumor ecosystem: a new insight to overall survival in stage I-III colorectal cancer. Chin J Cancer Res. 2022;34(1):40–52.
Grady WM. Genetic testing for high-risk colon cancer patients. Gastroenterology. 2003;124(6):1574–94.
Yang Y, Qu A, Wu Q, Zhang X, Wang L, Li C, Dong Z, Du L, Wang C. Prognostic value of a hypoxia-related microRNA signature in patients with colorectal cancer. Aging. 2020;12(1):35–52.
Xu H, Pan Y. A prognostic fibroblast-related risk signature in colorectal cancer. Aging. 2021;13(21):24251–70.
Hu X, Li YQ, Ma XJ, Zhang L, Cai SJ, Peng JJ. A risk signature with inflammatory and t immune cells infiltration in colorectal cancer predicting distant metastases and efficiency of chemotherapy. Front Oncol. 2019;9:704.
Zhuang Z, Cai H, Lin H, Guan B, Wu Y, Zhang Y, Liu X, Zhuang J, Guan G. Development and validation of a robust pyroptosis-related signature for predicting prognosis and immune status in patients with colon cancer. Journal of oncology. 2021;2021:5818512.
Annuk M, Zilmer M, Fellström B. Endothelium-dependent vasodilation and oxidative stress in chronic renal failure: impact on cardiovascular disease. Kidney Int Suppl. 2003;84:S50-53.
Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017:2525967.
Lee HT, Wu TH, Lin CS, Lee CS, Wei YH, Tsai CY, Chang DM. The pathogenesis of systemic lupus erythematosus—from the viewpoint of oxidative stress and mitochondrial dysfunction. Mitochondrion. 2016;30:1–7.
de Sá Junior PL, Câmara DAD, Porcacchia AS, Fonseca PMM, Jorge SD, Araldi RP, Ferreira AK. The roles of ROS in cancer heterogeneity and therapy. Oxid Med Cell Longev. 2017;2017:2467940.
Nakamura H, Takada K. Reactive oxygen species in cancer: current findings and future directions. Cancer Sci. 2021;112(10):3945–52.
Zuo L, Zhou T, Pannell BK, Ziegler AC, Best TM. Biological and physiological role of reactive oxygen species–the good, the bad and the ugly. Acta Physiol (Oxf). 2015;214(3):329–48.
Bae T, Hallis SP, Kwak M-K. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp Mol Med. 2024;56(3):501–14.
Li D, Yu Q, Wu R, Tuo Z, Wang J, Ye L, Shao F, Chaipanichkul P, Yoo KH, Wei W, et al. Interactions between oxidative stress and senescence in cancer: mechanisms, therapeutic implications, and future perspectives. Redox Biol. 2024;73: 103208.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385–95.
Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman C, Fridman WH, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17(1):218.
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–7.
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol Med. 2010;49(11):1603–16.
Gurer-Orhan H, Ince E, Konyar D, Saso L, Suzen S. The role of oxidative stress modulators in breast cancer. Curr Med Chem. 2018;25(33):4084–101.
Wang Z, Li Z, Ye Y, Xie L, Li W. Oxidative stress and liver cancer: etiology and therapeutic targets. Oxid Med Cell Longev. 2016;2016:7891574.
Borrego S, Vazquez A, Dasí F, Cerdá C, Iradi A, Tormos C, Sánchez JM, Bagán L, Boix J, Zaragoza C, et al. Oxidative stress and DNA damage in human gastric carcinoma: 8-Oxo-7’8-dihydro-2’-deoxyguanosine (8-oxo-dG) as a possible tumor marker. Int J Mol Sci. 2013;14(2):3467–86.
Saed GM, Diamond MP, Fletcher NM. Updates of the role of oxidative stress in the pathogenesis of ovarian cancer. Gynecol Oncol. 2017;145(3):595–602.
Klaunig JE. Oxidative Stress and Cancer. Curr Pharm Des. 2018;24(40):4771–8.
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discovery. 2013;12(12):931–47.
Li Q, Wu H, Cao M, Li H, He S, Yang F, Yan X, Zhang S, Teng Y, Xia C, et al. Colorectal cancer burden, trends and risk factors in China: a review and comparison with the United States. Chin J Cancer Res. 2022;34(5):483–95.
Colditz GA, Atwood KA, Emmons K, Monson RR, Willett WC, Trichopoulos D, Hunter DJ. Harvard report on cancer prevention volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention. Cancer Causes Control. 2000;11(6):477–488.
Liu L, Mo M, Chen X, Chao D, Zhang Y, Chen X, Wang Y, Zhang N, He N, Yuan X, et al. Targeting inhibition of prognosis-related lipid metabolism genes including CYP19A1 enhances immunotherapeutic response in colon cancer. J Exp Clin Cancer Res. 2023;42(1):85.
Zhu C, Sun Z, Li C, Guo R, Li L, Jin L, Wan R, Li S. Urocortin affects migration of hepatic cancer cell lines via differential regulation of cPLA2 and iPLA2. Cell Signal. 2014;26(5):1125–34.
He Q, Li Z, Yin J, Li Y, Yin Y, Lei X, Zhu W. Prognostic significance of autophagy-relevant gene markers in colorectal cancer. Front Oncol. 2021;11: 566539.
Sreevalsan S, Safe S. Reactive oxygen species and colorectal cancer. Curr Colorectal Cancer Rep. 2013;9(4):350–7.
Liebl MC, Hofmann TG. The role of p53 signaling in colorectal cancer. Cancers. 2021;13(9).
Ros-Martínez S, Navas-Carrillo D, Alonso-Romero JL, Orenes-Piñero E: Immunoscore: a novel prognostic tool. Association with clinical outcome, response to treatment and survival in several malignancies. Crit Rev Clin Lab Sci. 2020;57(7):432–443.
Feng DC, Zhu WZ, Wang J, Li DX, Shi X, Xiong Q, You J, Han P, Qiu S, Wei Q, et al. The implications of single-cell RNA-seq analysis in prostate cancer: unraveling tumor heterogeneity, therapeutic implications and pathways towards personalized therapy. Mil Med Res. 2024;11(1):21.
Song Y, Peng Y, Qin C, Wang Y, Yang W, Du Y, Xu T. Fibroblast growth factor receptor 3 mutation attenuates response to immune checkpoint blockade in metastatic urothelial carcinoma by driving immunosuppressive microenvironment. J Immunother Cancer. 2023;11(9).
Wang Q, Wang Z, Zhang Z, Zhang W, Zhang M, Shen Z, Ye Y, Jiang K, Wang S. Landscape of cell heterogeneity and evolutionary trajectory in ulcerative colitis-associated colon cancer revealed by single-cell RNA sequencing. Chin J Cancer Res. 2021;33(2):271–88.
Acknowledgements
The results of this study are based on the data from the TCGA dataset. We thank all the people who provided the data for this study.
Author information
Authors and Affiliations
Contributions
Yajie Wang: Literature collection, data analysis and manuscript writing. Lin Xiao: data analysis and software. Yisheng Pan: Project development and manuscript revised.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Xiao, L. & Pan, Y. Predictive role of oxidative stress-related genes in colon cancer: a retrospective cohort study based on The Cancer Genome Atlas. Discov Onc 15, 332 (2024). https://doi.org/10.1007/s12672-024-01216-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01216-5