Abstract
Background
Unlike human epidermal growth factor receptor 2 (HER2) amplification or exon 20 insertions, missense mutations in the extracellular domain (ECD), transmembrane domain (TMD), and intracellular domain (ICD) of the HER2 protein have been implicated as oncogenic in non-small cell lung cancer (NSCLC). However, their molecular subtypes, structural disparities, and clinical responses to current medical treatments, particularly HER2-targeted tyrosine kinase inhibitors (TKIs), remain unclear in NSCLC and warrant investigation.
Methods
A real-world observational ATLAS study was conducted to gather and analyze therapeutic outcomes of chemotherapy or TKIs for heterogeneous HER2 missense mutations in NSCLC. Computational models of typical ECD, TMD, and ICD mutations were utilized to explore their structural variances.
Results
We screened 37 eligible patients with HER2-activating missense mutations, of which 35 patients who had received chemotherapy or HER2-targeted TKIs as first-line therapy were available for response assessment. The median progression-free survival (PFS) for chemotherapy was 4.43 months (95% confidence interval [CI], 3.77–5.10), with an objective response rate (ORR) of 26.1% (6/23) and a disease control rate (DCR) of 17/23 (73.9%). The administration of afatinib, dacomitinib, and pyrotinib, HER2-targeted TKIs, achieved a median PFS of 4.65 months, with an ORR of 33.3% (4/12) and a DCR of 83.3% (10/12). Molecular modeling and computational simulations of ECD, TMD, and ICD mutations revealed their distinct structural characteristics.
Conclusion
In comparison to chemotherapy, HER2-targeted TKIs demonstrated similar activity and PFS benefits for HER2-activating missense mutations in NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The human epidermal growth factor receptor 2 (HER2, ERBB2) has been identified as an oncogenic driver in non-small cell lung cancer (NSCLC), with approximately 3% of patients with NSCLC harboring heterogeneous HER2 aberrations [1, 2]. As a receptor tyrosine kinase of the ErbB family, HER2 lacks a known ligand, and is instead activated by heterodimerization with other ErbB family members such as epidermal growth factor receptor (EGFR, HER1), HER3, and HER4 [1, 3]. Among its three functional forms, HER2 protein overexpression and gene amplification have been reported in 6–35% and 10–20% of NSCLC cases, respectively, whereas HER2 genomic alterations are documented to occur in only 2–4% [2, 4]. Exon 20 insertion has been reported to be the most common HER2-activating alteration in NSCLC, which tends to affect the ATP-binding pocket of the HER2 receptor, leading to steric hindrance against conventional HER2-targeted tyrosine kinase inhibitors (TKIs) [1, 5]. Additionally, several missense mutations in the extracellular domain (ECD, encoding amino acids 23–652), the transmembrane domain (TMD, encoding amino acids 653–675), and the intracellular domain (ICD, encoding amino acids 676–1255) of the HER2 protein have been identified as functional and oncogenic in NSCLC. These mutations can promote tumor proliferation and progression and may be targetable by TKIs, as supported by in vitro and in vivo evidence [6,7,8,9].
Various ECD or TMD missense mutations have been associated with sensitivity to HER2-targeted inhibitors in NSCLC [7,8,9]; however, it is not yet a validated hallmark to predict the response to HER2-targeted agents. An increasing number of HER2-activating missense mutations are being identified and described by multiplex next-generation sequencing (NGS) in NSCLC; however, current evidence regarding HER2-activating missense mutations in NSCLC consists mainly of case reports or studies with small sample sizes focusing on specific mutations due to their rare frequency.
Furthermore, there is a lack of research reporting comprehensive HER2 missense alteration subtypes and their clinical responses to HER2-targeted TKIs in NSCLC, with a focus on highlighting their molecular features to provide clinical reference as a benchmark. Therefore, our real-world ATLAS cohort study was conducted to evaluate the preliminary activity of HER2-targeted TKIs for heterogeneous HER2 missense alterations in metastatic NSCLC, aiming to reveal their discrepant structures.
2 Materials and methods
2.1 Patients and data collection
The medical records and clinical data of patients with metastatic NSCLC and HER2-activating missense mutations were retrospectively reviewed at the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences (Beijing), and Shandong Cancer Hospital and Institute (Jinan, Shandong Province) between September 2017 and April 2023. HER2-activating missense mutations were detected via NGS testing utilizing the Illumina sequencing system in institutional laboratories or qualified third-party genetic testing companies certified by the national quality system. Formalin-fixed, paraffin-embedded tissue samples or plasma were used. The last follow-up occurred on April 10, 2023. The requirement for informed consent was waived as this was a retrospective observational study.
2.2 Response assessment
Tumor response was evaluated as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) by analyzing computed tomography images of the chest and abdomen, and brain magnetic resonance imaging according to the Response Evaluation Criteria in Solid Tumors version 1.1. Progression-free survival (PFS) was considered the time from treatment initiation to the first evidence of disease progression or death from any cause. The objective response rate (ORR) was determined as the number of patients with confirmed CR and PR, whereas the disease control rate (DCR) was calculated as the proportion of confirmed CR, PR, and SD.
2.3 Computational structure modeling
The homology modeling procedure was conducted using the Molecular Operating Environment software (MOE, version 2020.01) “Homology Model” program, starting with a multiple sequence alignment of the primary structures. The three-dimensional (3D) structures of p.S310F and p.S310Y were generated through comparative modeling, using the HER2 ECD deposited in the RCSB Protein Data Bank [PDB, code: 3WLW] as a template. Similarly, the 3D structure of p.V659E was modeled using the TMD of EGFR and HER2 receptors deposited in the RCSB Protein Data Bank [PDB, code: 2KS1] as a template, whereas the structures of p.L755P and p.V842I were built based on the 2.25 Å resolution crystal structure of the kinase domain of human HER2 deposited in the RCSB Protein Data Bank [PDB, code: 3PP0].
2.4 Statistical analyses
Statistical analyses were performed using SPSS software, version 20.0 (IBM Corp., Armonk, NY, USA). Continuous variables were summarized using medians and ranges, and categorical variables were described by frequency and percentage. The Kaplan–Meier method was used to analyze PFS, and 95% confidence intervals (CIs) were estimated using the Cox proportional regression model. A p-value of < 0.05 was considered statistically significant. Survival curves were plotted using GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA).
3 Results
3.1 Patient characteristics and molecular subtypes
A total of 37 eligible patients with heterogeneous HER2-activating missense mutations were screened, of which 35 had received chemotherapy or HER2-targeted TKIs as first-line therapy and were available for response assessment. The clinicopathological characteristics of patients in this study was summarized in Table 1. All patients had lung adenocarcinomas, with 48.6% (n = 18) being females and 54.1% (n = 20) never-smokers. The majority underwent NGS testing on tumor tissue (n = 33, 89.2%). ECD mutations were identified in 13 patients (35.1%), including p.V308M (n = 1), p.S310F (n = 7), p.S310Y (n = 2), p.S335C (n = 1) in exon 8, and p.R456C (n = 2) in exon 12. TMD mutations were noted in five patients (13.5%), including p.V659E (n = 4) and V664F (n = 1) in exon 17. The remaining 19 patients (51.4%) harbored ICD mutations, including p.G727A in exon 18 (n = 1), p.L755P (n = 10), p.L755S (n = 2), and p.D769Y (n = 1) in exon 19, p.G776V (n = 1), p.R811Q (n = 1) in exon 20, and V842I (n = 1), p.T862A (n = 1), p.L841I plus L869R (n = 1) in exon 21.
3.2 Responses of first-line therapy for HER2 missense mutations
Among the 35 patients, 23 (62.2%) received first-line platinum-based chemotherapy, including chemotherapy alone (n = 5), chemotherapy in combination with anti-vascular endothelial growth factor (VEGF) antibody bevacizumab (n = 10), chemotherapy in combination with programmed cell death protein 1 (PD-1) inhibitor (n = 6), or chemotherapy in combination with bevacizumab and PD-1 inhibitor (n = 2). The median PFS for chemotherapy-based options was 4.43 (95% CI 3.77–5.10) months, with an ORR of 26.1% (6/23) and a DCR of 73.9% (17/23). Detailed chemotherapy regimen, genetic mutation and response for each case was summarized in Table 2. HER2-targeted TKIs (afatinib, dacomitinib, pyrotinib) were administered to another 12 patients (32.4%) as first-line therapy. They achieved a median PFS of 4.65 months (P = 0.527, Fig. 1A), with an ORR of 33.3% (4/12) and a DCR of 83.3% (10/12) compared with chemotherapy-based option. Table 3 summarized the targeted outcomes of heterogeneous HER2 missense mutations, and a swimmer plot depicting the PFS benefit of HER2-targeted TKIs is shown in Fig. 1B.
3.3 Efficacy of HER2-targeted TKIs for HER2 missense mutations
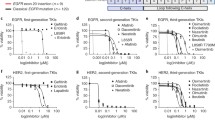
Among the six patients with p.S310F mutation, one patient exhibited intrinsic PD (PFS of 1.9 months) to afatinib, whereas another exhibited SD, with an ongoing PFS of 34.7 months to afatinib in the first-line setting. The remaining four patients responded with SD to afatinib or pyrotinib, with PFS ranging between 2.4 and 5.4 months (Fig. 2A). One patient with the p.S310Y mutation exhibited SD (PFS of 3.9 months) to dacomitinib and SD (PFS of 2.3 months) to pyrotinib. Similarly, another patient with the p.S310Y mutation exhibited SD (PFS of 3.8 months) to first-line pyrotinib but experienced rapid PD (PFS of 0.9 months) to afatinib (Fig. 2B). For the three patients with the p.V659E alteration, two received pyrotinib (PFS of 2.4 months) and afatinib (PFS of 2.2 months), both achieving SD as the best response. Dacomitinib (PFS of 9.4 months) and pyrotinib (PFS of 6.0 months, Fig. 2C) were administered to the third patient, also with SD as the best response.
Case series revealing targeted responses to HER2-TKIs of variable HER2 missense alterations. S310F (A) and S310Y (B) in exon 8 of ECD, V659E in exon 17 of TMD (C), and kinase domain alterations in the ICD, including G727A in exon 18 (D), L755P in exon 19 (E), G776V in exon 20 (F), and V842I in exon 21 (G)
For the nine patients with ICD missense alterations, diverse alteration subtypes in the kinase domain spanning exon 18–21 were observed, with variable responses to HER2-targeted TKIs. A patient with the exon 18 p.G727A mutation exhibited PR to pyrotinib, with a PFS of 9.8 months (Fig. 2D). Another patient harboring the exon 19 p.L755P mutation exhibited PR and PFS of 8.5 months to pyrotinib (Fig. 2E). A patient with exon 20 p.G776V mutation achieved significant PFS benefit of 17.5 months to pyrotinib (Fig. 2F). However, a patient harboring exon 21 p.V842I mutation experienced intrinsic PD to both pyrotinib (PFS of 0.9 month, Fig. 2G) and afatinib (PFS of 0.8 month).
3.4 Structural analysis
In the crystal structure of the wild-type HER2 receptor, the amino acid residue Ser310 is situated in the ECD, exhibiting an H-bond interaction with Thr290. However, in the p.S310F conformation (Fig. 3A), Phe310 lacks interaction with Thr290. Similarly, in the p.S310Y conformation (Fig. 3B), Tyr310 also does not interact with Thr290. The homology structures of the hydrophilic residue Glu659 in the TMD of the EGFR/HER2 dimer are depicted in Fig. 3C. Residue Leu755 in the kinase domain of HER2 is proximal to the Hyd1 region (marked in orange, Fig. 3D), an important hydrophobic region encoding amino acids Ala751-Ile752-Lys753. Residues within Hyd1 engage in hydrophobic contact with the adenine ring of ATP, forming a hydrophobic pocket. Homology modeling revealed that both leucine (Leu755) and proline (Pro755) are hydrophobic amino acids, exhibiting little difference in 3D structural conformation (Fig. 3E). However, Val842 is in close proximity to the catalytic subunit (marked in red, Fig. 3D), encoding amino acids His843 to Leu852, and Val842 interacts with Asp904 and His901 via several H-bonds (Fig. 3F), contributing to the stability of the HER2 protein. No interactions between the mutated Ile842 and Asp904 and His901 were observed (Fig. 3G). The conformation of V842I is less stable than that of wild-type Val842 because of the absence of molecular interaction, potentially resulting in structural changes in the HER2 protein. Moreover, the V842I alteration eliminates the interaction between Val842 itself and other protein residues, contributing to modifications in amino acid positions of the catalytic unit, such as Asp845, Leu846, and Arg849 (Fig. 3H).
Crystal structures and molecular interactions between S310F (A) and S310Y (B). Homology models of V659E (C) in the TMD of EGFR/HER2 dimer. Residue Leu755 and Val842 in the kinase domain of HER2 (D). Homology modelling of Leu755 and Pro755 (E). Val842 close to the catalytic subunit interacts with Asp904 and His901 via H-bonds (F), and there was no interaction between Ile842 and Asp904 or His901 (G). Modification in amino acid positions of the catalytic unit induced of V842I mutation (H)
4 Discussion
This real-world ATLAS study offers valuable insights into the molecular signature and clinical activity of chemotherapy or HER2-targeted TKIs against uncommon HER2-activating missense mutations in NSCLC. Currently, we present the most comprehensive atlas for HER2-activating missense mutations in NSCLC (Fig. 4), encompassing molecular subtypes of various missense mutations across each exon of the HER2 receptor in NSCLC, documented or reported in datasets such as Cosmic, cBioPortal, and Foundation Medicine sequencing [9]. Certain types of HER2 missense alterations in NSCLC have been identified as oncogenic and have shown sensitivity to HER2-targeted inhibitors [9,10,11,12,13,14,15,16,17]. Our observations demonstrate that HER2-targeted TKIs, including afatinib, dacomitinib, and pyrotinib, exhibit similar PFS (median, 4.65 vs. 4.43 months) and ORRs (33.3% vs. 26.1%) compared to chemotherapy for these alterations as first-line therapy, respectively.
HER2 somatic alterations have been reported across various human cancer types and are linked with activating functions that drive oncogenesis akin to gene amplification and HER2 protein overexpression, which may similarly confer drug sensitivity to HER2-targeted TKIs [18,19,20]. In NSCLC, exon 20 insertions in the HER2 kinase domain have been extensively studied regarding their molecular features and responses to clinical treatment [1, 2, 5]. However, other heterogeneous missense mutations in NSCLC in different regions of HER2, such as ECD, TMD, and ICD, have received less attention and investigation regarding their potential for activating HER2 and their responses to currently available HER2-directed TKIs. The most common ECD mutation, p.S310F, is a well-known activating alteration promoting HER2 noncovalent dimerization and mediating HER2 hyperphosphorylation [8, 21]. Few reports on p.S310F have mentioned favorable responses to trastuzumab and lapatinib [22, 23]. Evidence has also validated that p.S310F induces structural changes in the HER2 extracellular subdomain II in vitro and forms an active heterodimer with EGFR, thereby abolishing reactivity to pertuzumab [2]. Another ECD mutation, p.S310Y, has been identified in patients with NSCLC [8, 24], with reported sensitivity to afatinib in lung adenocarcinoma [12]. Functional analysis suggests that HER2 ECD mutants p.S310F and p.S310Y can be activated by mechanisms involving C-terminal phosphorylation elevation and formation of disulfide-linked dimers, leading to hydrophobic interactions between the newly introduced 310F or 310Y and neighboring molecules, thus promoting noncovalent dimerization and HER2 kinase activation, which may benefit from HER2-targeted therapy [8].
In our ECD mutation cohort, five patients harboring p.S310F exhibited SD to afatinib or pyrotinib, with one patient exhibiting an ongoing PFS of 34.7 months with first-line afatinib therapy. Additionally, two patients harboring the p.S310Y mutation exhibited SD to dacomitinib or pyrotinib. These results suggest that pan-ErbB TKIs exhibit promising activity for HER2 ECD mutations in NSCLC. To the best of our knowledge, this is the first report on dacomitinib and pyrotinib activities for p.S310F and p.S310Y mutations. Our structural analysis provides meaningful evidence regarding p.S310F and p.S310Y mutations and offers supplementary insights into the molecular hydrophobicity and interactions of HER2 ECD missense mutations. Additionally, we report a valuable case with the p.R456C mutation in exon 12, another rare ECD missense alteration which has not been previously reported, achieving a favorable PFS of 20 months on afatinib.
Furthermore, the ErbB family TMD is crucial for receptor activation, affecting downstream signaling activity independently of kinase domain mutations [25]. Typical HER2 TMD mutations, such as p.V659E, are well-known NSCLC oncogenic drivers [13, 26, 27]. The largest research cohort of more than 8000 lung adenocarcinoma samples to date reported the detection of 0.15% (15/8551) of HER2 TMD mutations at amino acid residues V659 or G660 in NSCLC, including p.V659E, p.V659D, p.G660D, and p.G660R, along with other non-V659/G660 TMD mutations, including p.V664F, p.V665M, and p.I675M [7]. Ou et al. performed structural analysis, revealing that V659/G660 TMD mutations stabilized HER2 homodimerization and heterodimerization to maintain HER2 in the active conformation, with treatment using afatinib resulting in durable clinical responses in three of four patients [7]. A Chinese multi-center cohort study also revealed the comprehensive profiles and real-world evidence of HER2 TMD mutation treatment in NSCLC, with a total prevalence of 0.18% (14/7812) and 0.14% (11/7812) for the p.V659E alteration. This study indicated that TMD mutations were associated with more advanced stages (p < 0.001) and poorer overall survival (median, 10.0 vs. 61.6 months, hazard ratio = 7.9, p < 0.001) than non-TMD mutations [28]. Favorable PFS outcomes with targeted therapy, including afatinib (up to 16 months), and better responses to pyrotinib were noted among cohort patients, suggesting that pyrotinib effectively inhibits the p.V659E mutation. Structural analysis of binding affinity subsequently demonstrated increased binding ability to pyrotinib and afatinib toward the p.V659E mutation [28]. In our study, which included three patients harboring p.V659E, our data correlated well with the abovementioned evidence, further implying that, in addition to afatinib and pyrotinib, dacomitinib is a promising TKI candidate for the p.V659E mutation. We observed that the p.V659E mutation altered the hydrophobicity of residue Val659, which may explain the discrepant binding affinity to the abovementioned HER2-targeted TKIs.
Among HER2 ICD alterations in NSCLC, the in-frame insertion in exon 20 (A775_G776insYVMA) is the most frequent subtype [1, 2, 6]. Several studies have revealed that exon 20 insertions exhibit relatively poor responses to traditional pan-ErbB TKIs, including afatinib, dacomitinib, and neratinib, with low ORRs of 3.8–11.5% and PFS ranging from 3 to 5.5 months [16, 29, 30]. The ZENITH20-2 study reported a median PFS of 5.5 months and an improved ORR of 35.1% in patients with NSCLC harboring HER2 exon 20 insertions treated with the novel pan-ErbB TKI poziotinib [31]. A significantly improved PFS (median, 8.2 months) and ORR of 55% was reported in patients with NSCLC and HER2 mutations treated with trastuzumab deruxtecan (T-DXd, DS-8201) in the DESTINY-Lung01 study [32]. Prospective studies have increasingly focused on HER2 exon 20 insertions in advanced NSCLC; however, little is known regarding the targeted outcomes of pan-ErbB TKIs for the uncommon HER2 ICD missense alterations in real-world settings. Our PEARL study revealed a median PFS of 5.8 months, an ORR of 23.0%, and a DCR of 85.1% with pyrotinib among HER2-mutated patients with NSCLC. Those with HER2 missense mutations exhibited a notable PFS benefit (median, 12.2 vs. 6.8 vs. 5.2 months) compared with HER2 amplification and exon 20 non-YVMA insertions, respectively [33]. Based on a single-arm, phase II study on HER2-mutated advanced NSCLC, pyrotinib facilitated a median PFS of 6.9 months and an ORR of 30% as second-line or above therapy. Several patients with HER2 kinase domain missense mutations, including p.G776R, p.G776C, p.V777L, p.L755P, were enrolled, with an ORR of 25.0% in four patients with the p.L755P mutation [34].
In NSCLC, exon 18 p.G727A, exon 20 p.G776V, and exon 21 p.V842I mutations in the HER2 receptor kinase domain have been recorded in the Foundation Medicine or cBioPortal dataset and recognized as oncogenic [9]; however, their responses to HER2 inhibitors remain unclear. Moreover, while the p.L775P and p.L755S missense mutations have been considered oncogenic and sensitive to neratinib, poziotinib, and pyrotinib [15, 16, 34], their responses to afatinib and antitumor capability confirmed by pyrotinib require further study. Our ATLAS cohort study provided initial evidence of activity with HER2-targeted TKIs for these HER2 rare missense mutations. We observed that both the p.G727A and p.G776V mutations exhibited favorable responses to pyrotinib, with significant PFS benefits of 9.8 and 17.5 months, respectively. However, the p.V842I mutation exhibited de novo drug resistance to both pyrotinib and afatinib. This resistance might be attributed to its location near the catalytic subunit and the lack of molecular interaction between Ile842 and other residues, resulting in modifications in amino acid positions of the catalytic unit and structural changes in the HER2 protein, as indicated by in silico structural analysis. In our cohort, patients with the p.L755P and p.L755S mutations treated with afatinib or pyrotinib exhibited favorable responses and PFS benefits, similar to what has been previously reported [34]. Furthermore, it was found that both residue Leu755 and Pro755 were hydrophobic amino acids, with little difference in their activity to TKI binding and 3D structural conformation.
HER2-mutant lung cancers have a clinical course with a high incidence of brain metastases [35]. One of the reason is the absence of effective, targeted therapies for HER2 mutations, and currently the first-line standard recommendation for the treatment of lung cancer with HER2 mutations still remains cytotoxic chemotherapy. In addition, HER2-mutant cancers are associated with increased expression of the chemokine receptor C-X-C chemokine receptor type 4 (CXCR4). CXCR4 and its ligand, stromal-derived-factor-1 (SDF-1; also called CXCL12), may drive metastatic trafficking to the brain [36, 37]. The frequency of brain metastases at diagnosis was similar in NSCLC patients carrying HER2 mutations (19%), compared to patients with KRAS (24%) and EGFR mutations (31%) [35]. However, lung cancer patients with HER2 mutations developed more brain metastases during treatment than patients with KRAS (28% vs. 8%, hazard ratio [HR] 5.2, P < 0.001) and EGFR mutations (28% vs 16%, HR 1.7, P = 0.06) [35]. Yang et al. also demonstrated that HER2 exon 20 YVMA insertion is associated with a higher incidence of lifetime brain metastasis, with estimated 12-month brain metastasis incidence as 40.2% compared with 3.6% in the non-YVMA group in patients with advanced NSCLC and HER2 kinase domain mutations [38]. In our cohort study, among the total 37 patients harboring HER2-activating missense mutations, only five (14%) presented baseline brain metastasis. After the failure of first-line therapy, eight patients occurred brain metastasis, with a brain metastasis frequency of 35%, which is in accordance with observations discussed above.
T-DXd is currently the sole approved HER2-targeted therapy for previously treated NSCLC patients with HER2 mutations. The randomized, blinded multicenter phase II trial DESTINY-Lung02 showed considerable and enduring antitumor responses of T-DXd in HER2-mutated NSCLC patients, with ORR of 49% and 56% at doses of 5.4 and 6.4 mg/kg, regardless of HER2 mutation type, amplification status, and prior treatment [39]. In several countries, T-DXd, rather than HER2-targeted TKIs, has become the standard choice for second-line treatment in NSCLC patients with HER2 mutations. Unfortunately, none of the patients with HER2-activating missense mutations involved in this study were treated with T-DXd. The accessibility of T-DXd in the mailand of China, financial considerations from patients and their family members, and the current cognition of HER2-activating missense mutations both from lung cancer patients and doctors are all factors that might explain for this status.
Sugimoto et al. had reported plasma cell-free DNA (cfDNA) sequencing in patients with NSCLC showed relatively high sensitivity for detecting gene mutations but low sensitivity for gene fusions and MET exon 14 skipping, with a positive percent agreement of plasma cfDNA sequencing compared with tissue DNA and RNA assays were 77% (EGFR, 78%; KRAS, 75%; BRAF, 85%; HER2, 72%) and 47% (ALK, 46%; RET, 57%; ROS1, 18%; MET, 66%), respectively [40]. Plasma cfDNA sequencing could be useful for detecting oncogenic alterations only when tissue assay is unavailable, and it could not fully replace tissue assays for oncogenic alterations detection, especially when the quality and quantity of tissue samples are acceptable for genomic analysis. In this cohort study, four patients performed liquid biopsy using circulating cfDNA owing to the inadequate tumor tissue or the will of non-invasive testing. They were detected to carry p.L755P, p.V659E, p.S310Y, and p.L841I with p.L869R missense mutations, respectively. To some degree, liquid biopsy for HER2-mutant lung cancer using circulating cfDNA is a reasonable alternative.
Despite the ATLAS study investigated the clinical activity of currently available HER2-targeted TKIs for heterogeneous HER2 missense mutations in NSCLC, along with valuable evidence from in silico structural analysis, several limitations must be noted. Firstly, this was a retrospective real-world study prone to selection bias. Additionally, the small sample size of patients with HER2 missense mutations prevented us from further investigating molecular variants and their precise activity toward HER2-targeted TKIs. The structural differences and molecular interactions were explained with regard to specific missense subtypes; however, this was an exploratory analysis based on computational structure and dynamics simulation and may not fully represent all possible reasons. Further clinical evidence, including cell lines and patient-derived xenograft models, is warranted to corroborate our findings and draw clear conclusions.
In conclusion, compared to conventional chemotherapy, currently available HER2-targeted TKIs exhibited similar efficacy and reasonable activity for patients with NSCLC harboring HER2-activating missense mutations. Awareness of these extremely rare but oncogenic HER2 missense alterations may advance promising targeted therapy in NSCLC for this entity.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012;18:4910–8.
Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31:1997–2003.
Oxnard GR, Binder A, Jänne PA. New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31:1097–104.
Peters S, Zimmermann S. Targeted therapy in NSCLC driven by HER2 insertions. Transl Lung Cancer Res. 2014;3:84–8.
Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65:1642–6.
Notsuda H, Bradbury PA, Tsao MS. HER2 transmembrane domain mutations: rare new target for non-small cell lung cancer therapy. J Thorac Oncol. 2017;12:422–4.
Ou SI, Schrock AB, Bocharov EV, et al. HER2 transmembrane domain (TMD) mutations (V659/G660) that stabilize homo—and heterodimerization are rare oncogenic drivers in lung adenocarcinoma that respond to afatinib. J Thorac Oncol. 2017;12:446–57.
Greulich H, Kaplan B, Mertins P, et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012;109:14476–81.
Zeng J, Ma W, Young RB, et al. Targeting HER2 genomic alterations in non-small cell lung cancer. J Natl Cancer Cent. 2021;1:58–73.
Li BT, Shen R, Buonocore D, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018;36:2532–7.
Gao Y, Zheng A, Zhu X, et al. Clinical benefit from afatinib in an advanced squamous cell lung carcinoma patient harboring HER2 S310Y mutation: a case report. Onco Targets Ther. 2018;11:8705–10.
Wang J, Wen Y, Ding G, et al. Efficacy generated by afatinib in a lung adenocarcinoma patient harboring HER2 S310Y mutation. Cancer Biol Ther. 2018;19:450–3.
Serra V, Vivancos A, Puente XS, et al. Clinical response to a lapatinib-based therapy for a Li-Fraumeni syndrome patient with a novel HER2 V659E mutation. Cancer Discov. 2013;3:1238–44.
Yamamoto H, Toyooka S, Ninomiya T, et al. Therapeutic potential of afatinib for cancers with ERBB2 (HER2) transmembrane domain mutations G660D and V659E. Oncologist. 2018;23:150–4.
Robichaux JP, Elamin YY, Vijayan RSK, et al. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell. 2019;36:444–57.
Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554:189–94.
Zhang P, Nie X, Wang B, et al. Combined therapy with osimertinib and afatinib in a lung adenocarcinoma patient with EGFR T790M mutation and multiple HER2 alterations after resistance to icotinib: a case report. Thorac Cancer. 2018;9:1774–7.
Connell CM, Doherty GJ. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open. 2017;2: e000279.
Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–87.
Shin JW, Kim S, Ha S, et al. The HER2 S310F mutant can form an active heterodimer with the EGFR, which can be inhibited by cetuximab but not by trastuzumab as well as pertuzumab. Biomolecules. 2019;9:629.
Petrelli F, Tomasello G, Barni S, et al. Clinical and pathological characterization of HER2 mutations in human breast cancer: a systematic review of the literature. Breast Cancer Res Treat. 2017;166:339–49.
Jasra S, Opyrchal M, Norton L, et al. A rare case of S310F somatic ERBB2 mutation in a HER2-nonamplified breast cancer. Clin Breast Cancer. 2017;17:e37–41.
Vornicova O, Hershkovitz D, Yablonski-Peretz T, et al. Treatment of metastatic extramammary Paget’s disease associated with adnexal adenocarcinoma, with anti-HER2 drugs bBased on genomic alteration ERBB2 S310F. Oncologist. 2014;19:1006–7.
Kan Z, Jaiswal BS, Stinson J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–73.
Bocharov EV, Lesovoy DM, Pavlov KV, et al. Alternative packing of EGFR transmembrane domain suggests that protein-lipid interactions underlie signal conduction across membrane. Biochim Biophys Acta. 2016;1858:1254–61.
Yamamoto H, Higasa K, Sakaguchi M, et al. Novel germline mutation in the transmembrane domain of HER2 in familial lung adenocarcinomas. J Natl Cancer Inst. 2014;106:djt338.
Wang R, Zhang Y, Pan Y, et al. Comprehensive investigation of oncogenic driver mutations in Chinese non-small cell lung cancer patients. Oncotarget. 2015;6:34300–8.
Jia Z, Xing J, Li J, et al. HER2 transmembrane domain mutation: comprehensive characteristics and real-world evidence of treatment response in Chinese lung adenocarcinoma. Transl Lung Cancer Res. 2021;10:1383–96.
Dziadziuszko R, Smit EF, Dafni U, et al. Afatinib in NSCLC with HER2 mutations: results of the prospective, open-label phase II NICHE trial of European thoracic oncology platform (ETOP). J Thorac Oncol. 2019;14:1086–94.
Kris MG, Camidge DR, Giaccone G, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol. 2015;26:1421–7.
Socinski M, Cornelissen R, Garassino MC, et al. LBA60 ZENITH20, a multinational, multi-cohort phase II study of poziotinib in NSCLC patients with EGFR or HER2 exon 20 insertion mutations. Ann Oncol. 2020;31:S1188.
Li BT, Smit EF, Goto Y, et al. DESTINY-Lung01 trial investigators trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386:241–51.
Yang G, Hao X, Hu J, et al. Pyrotinib in HER2 heterogeneously mutated or amplified advanced non-small cell lung cancer patients: a retrospective real-world study (PEARL). J Natl Cancer Cent. 2021;1:139–46.
Zhou C, Li X, Wang Q, et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, phase II study. J Clin Oncol. 2020;38:2753–61.
Offin M, Feldman D, Ni A, et al. Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers. Cancer. 2019;125:4380–7.
Hinton CV, Avraham S, Avraham HK. Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clin Exp Metastasis. 2010;27:97–105.
Lee BC, Lee TH, Avraham S, et al. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2:327–38.
Yang S, Wang Y, Zhao C, et al. Exon 20 YVMA insertion is associated with high incidence of brain metastasis and inferior outcome of chemotherapy in advanced non-small cell lung cancer patients with HER2 kinase domain mutations. Transl Lung Cancer Res. 2020;10:753–65.
Goto K, Goto Y, Kubo T, et al. trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small-cell lung cancer: primary results from the randomized, Phase II DESTINY-Lung02 trial. J Clin Oncol. 2023;41:4852–63.
Sugimoto A, Matsumoto S, Udagawa H, et al. A large-scale prospective concordance study of plasma—and tissue-based next-generation targeted sequencing for advanced non-small cell lung cancer (LC-SCRUM-Liquid). Clin Cancer Res. 2023;29:1506–14.
Acknowledgements
We would like to take the opportunity to thank the patients, their families, and all of the research members in this study. We appreciate the linguistic assistance provided by TopEdit (www.topeditsci.com) during the preparation of this manuscript.
Funding
This study was supported by the “Wu Jieping Medical Foundation” (320.6750.2023-17-24).
Author information
Authors and Affiliations
Contributions
Conceptualization: GY; Methodology: GY, RL; software: GY, RL; Validation: GY, PL; Formal analysis: GY, RL; Investigation: GY, PL, RL, YY, YW; Resources: YY, XT; Data curation: GY, PL, RL, YY, YW, HM; writing—original draft preparation: GY, RL, PL; writing—review and editing: GY, XT; Visualization: GY, PL; Supervision: XT; Project administration: GY, PL. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute, and National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, and was carried out in compliance with the Declaration of Helsinki Principles. As a retrospective study, it was exempted from obtaining patients’ informed consent without therapeutic intervention.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, G., Liu, R., Li, P. et al. Clinical and structural insights into the rare but oncogenic HER2-activating missense mutations in non-small cell lung cancer: a retrospective ATLAS cohort study. Discov Onc 15, 285 (2024). https://doi.org/10.1007/s12672-024-01154-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01154-2