Abstract
Molecular testing is performed upon diagnosis of non-small cell lung cancer (NSCLC) because of the large success of targeted therapies for oncogenic mutations. Epidermal growth factor receptor (EGFR) mutations are the most commonly identified mutation in NSCLC, and EGFR exon 20 insertion mutations (exon20ins) are the third most common mutation in EGFR following EGFR exon 19 deletions and exon 21 L858R mutations. EGFR exon20ins have regularly demonstrated resistance to classical EGFR inhibition. Two treatments—mobocertinib and amivantamab—have recently been the first drugs to be approved by the US Food and Drug Administration (FDA) for treatment of lung cancers with these mutations following platinum-based therapy. Research surrounding these two drugs demonstrates strong efficacy, but with an intense array of side effects. Another targetable driver mutation is the human epidermal growth factor receptor 2 (HER2) exon20ins, representing approximately 2–3% of NSCLC patients. This mutation has been heavily studied in vitro as well as clinically, and trastuzumab deruxtecan was just recently granted accelerated FDA approval based on the high efficacy demonstrated in the Destiny-Lung01 study. However, similar to their EGFR counterparts, HER2 inhibitors also have evidence of toxicity in clinical studies. In this paper, we discuss the limited response of EGFR and HER2 exon20ins to a wide range of standard treatment regimens, such as platinum-based chemotherapy and classic EGFR tyrosine kinase inhibitors, as well as immunotherapy. We also review recently approved and upcoming targeted therapeutic options, considering what research is presently being done regarding efficacy and the reduction of side effects, as well as the agents’ risks and benefits for incorporation into an approved treatment regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Exon 20 insertion mutations in epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) are resistant to traditional EGFR-targeting agents. |
Recently, two agents (amivantamab and mobocertinib) have been approved for EGFR exon 20 insertion mutations. |
Trastuzumab deruxtecan was also recently Food and Drug Administration (FDA) approved for non-small cell lung cancer with HER2 mutations. |
Novel agents to improve upon the efficacy and toxicity profile are being developed in the exon 20 insertion mutation space. |
1 Introduction
Lung cancer affects over 2 million people annually, with non-small cell lung cancer (NSCLC) accounting for 82% of lung cancer cases [1, 2]. Much is known about the epidemiology of NSCLC, including how varying mutations impact prognosis and outcome, and as a result, targeted therapies have been developed for several oncogenic mutations.
Epidermal growth factor receptor (EGFR) mutations are the most commonly identified mutation in NSCLC, occurring in 10–15% of all NSCLC cases [3]. Carcinogenic mutations typically mutate the EGFR protein to a constitutively active state. EGFR mutations are predominantly found in females, never-smokers, and Asians with adenocarcinoma [4]. The two most common EGFR alterations, or classic EGFR mutations, are exon 19 deletions and an L858R point mutation on exon 21; together, they make up about 85–90% of EGFR mutations [3]. Tyrosine kinase inhibitors (TKIs) have been designed to target active EGFR proteins, selectively targeting the carcinogenic mutations. Osimertinib has seen the most recent approvals for NSCLC harboring classic EGFR mutations or exon 19 deletions and exon 21 L858R mutations; it is an irreversible inhibitor that covalently binds to the EGFR protein at Cys797, blocking the adenosine triphosphate (ATP) binding site [5]. It was initially approved for treatment of those who developed the T790M escape mutation post earlier generation EGFR TKIs [13]. However, after the FLAURA study, which compared osimertinib to both gefitinib and erlotinib, demonstrated an increase in progression-free survival (PFS) as well as overall survival (OS), osimertinib is now considered the preferred frontline treatment for patients with metastatic NSCLC harboring EGFR mutations [6]. Additionally, the ADAURA study recently compared treatment with osimertinib versus placebo following surgical resection of early stage NSCLC, and it showed a significant increase in disease-free survival, with improvements increasing with disease stage [7].
2 Diagnostic Testing
At any new diagnosis of advanced NSCLC, molecular testing is paramount for a full clinical picture of the disease in order to understand how best to proceed with treatment. Current testing numbers are not fully known, but a survey from 2020 estimates that approximately 61% of physicians consistently order molecular testing for newly diagnosed advanced-stage NSCLC [8].
2.1 Diagnosis of HER2 Aberrations
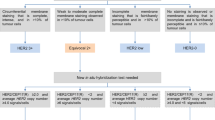
Human epidermal growth factor receptor (HER2) alterations are diagnosed by (1) fluorescence in situ hybridization (FISH) for HER2 amplifications, (2) immunohistochemical (IHC) staining for HER2 overexpression, or (3) next-generation sequencing (NGS) for mutations [9].
2.2 Diagnosis of EGFR Aberrations
EGFR mutations generally consist of point mutations or variably sized mutations below the threshold of testing for karyotype, FISH, or IHC staining [10]. Thus, mutational testing can be done via either liquid biopsy or tumor tissue sequencing. Liquid biopsy testing is primarily only recommended for advanced or metastatic disease, since it measures cell-free DNA, which is more prevalent in metastatic cancers. However, liquid biopsies still show a 30% false-negative rate, so they should not be the standalone method of analysis. Guardant360 (Guardant, CA) is an NGS-based device using cell-free DNA from plasma to identify NSCLC patients who may benefit from treatment with osimertinib. Validation testing showed a specificity of > 99.9%, with sensitivity of 85.0%, compared to sensitivity of 80.7% in tissue-based samples [11].
Polymerase chain reaction (PCR) has a high sensitivity for detecting only those assays for which the test was designed. PCR can detect mutant allelic frequencies as low as 1% [10]. Tissue analysis by NGS is currently the gold standard [12], though results may take weeks or more to report. NGS has an advantage of providing detection of (1) single nucleotide variants, (2) copy number variants, and (3) rearrangements in multiple genes simultaneously [10]. NGS can detect allelic frequency down to 0.1% [11]. In one analysis of 31 EGFR samples, the concordance rate between PCR and NGS was 90.3%, with more aberrations detected via NGS [13]. One retrospective analysis found use of PCR decreased from 100% in 2011 to 6.5% by 2020, while the rate of NGS increased from 0% in 2011 to 64.5% in 2020 [14]. Testing was sampled from tissue in 84.9% of EGFR exon 20 insertion mutation (exon20ins) cases versus blood in 17.7%. Across all assays, the median time from diagnosis to EGFR exon20ins result was 23 days (28 days for NGS vs 12 days for PCR), with a median laboratory turnaround time of 9 days (11 days for NGS and 8 days for PCR).
3 Epidemiology and Structure
3.1 EGFR Exon 20 Insertions: Epidemiology and Structure
Although EGFR exon 19 deletions and L858R point mutations represent the majority of EGFR mutations, exon20ins make up 4% of EGFR mutants. EGFR exon20ins are generally mutually exclusive of other mutations [15]. In contrast to classic mutations, exon20ins do not sensitize the kinase domain to EGFR TKIs, thus acting as resistance mutations [16,17,18,19]. To elucidate the mechanism behind this, Yasuda et al. developed the first crystal structure of an exon20ins EGFR mutation, D770_N771insNPG [19]. The crystal structure revealed an active conformation with the C-helix in an inward position, forming a rigid and inflexible structure that locks the EGFR molecules in active conformation without ligand binding [19].
As a result of blocking the binding domain, patients with EGFR exon20ins have shorter OS than patients with common EGFR mutations, due to a lack of targeted therapeutic options [4]. This is explained by the structural changes in the EGFR protein—the insertion, typically around codons 762–774, keeps the protein in its active confirmation [20]. These mutations typically represent insertions ranging from 3 to 21 base pairs. The insertion sequences were highly variable, with the most common variant (V769_D770insASV) found in 22% of cases [4]. Since ATP binding is not needed to shift these proteins into the active state, this renders traditional TKIs clinically useless against tumors expressing EGFR with exon20ins. Furthermore, the traditional TKIs, including osimertinib, are chemically unable to bind to the enzyme when in its active state. The median survival of 1086 patients with EGFR exon20ins receiving either TKIs or chemotherapy was 16 months [4].
This had been a major area of unmet need until very recently, when we finally saw the approval of mobocertinib and amivantamab.
3.2 HER2 Exon 20 Insertions: Epidemiology and Structure
HER2 mutations are found in 2–3% of lung adenocarcinoma patients [21,22,23,24,25]. In a report from the Cancer Genome Atlas, HER2 mutations were seen in 4% of NSCLC patients [26]. Most of these mutations (90%) occur as an insertion mutation within the exon 20 frame, with duplication of A775_G776insYVMA being the most common [21,22,23,24]. HER2 exon20ins result in constitutive activation of the receptor with downstream effects on AKT/MEK pathways [27]. Simulations show that HER2 exon20ins restrict HER2 kinase to its active state, resulting in ligand-independent kinase activation [28]. Thus, they have been classified as driver oncogenic mutations. HER2 exon20ins are associated with women and never-smokers [21, 23]. HER2 mutation is also considered a mechanism for resistance to TKIs. Arcila et al. reported OS of 19 months for HER2-mutated NSCLC patients compared to 30 months for EGFR-mutated NSCLC patients on any treatment [21]. Another study confirmed the OS to be around 24 months [29].
4 Response to Standard Therapies
4.1 Chemotherapy
Currently, the first-line standard-of-care treatment for EGFR exon20ins and HER2 exon20ins patients is platinum-based chemotherapy. In a case series of 27 NSCLC patients with EGFR exon20ins, 67% received chemotherapy, 94% in the first-line setting [30]. Most patients (17/18) received carboplatin plus pemetrexed. The objective response rate (ORR) to chemotherapy was 39% (95% confidence interval [CI] 16–61), with PFS of 7.1 months (95% CI 6.3–13.7). The OS was 3.2 years (95% CI 1.92–not reached [NR]). One-third (6/18) of patients had bevacizumab added to their regimen, with similar ORR and PFS (50% and 6.2 months, respectively). The authors concluded that EGFR exon20ins NSCLC has a similar response to chemotherapy as wild-type EGFR NSCLC, which has shown an ORR of 30%, with a median PFS of 5–6 months in prior literature [31,32,33]. A review of 104 Chinese patients with EGFR exon20ins receiving first-line platinum-based chemotherapy found an ORR of 19.2%, with median PFS of 6.4 months (95% CI 5.7–7.1) [34]. A review of 77 patients receiving pemetrexed-based first-line chemotherapy found an ORR of 41.6% [35]. The authors also showed that pemetrexed-based chemotherapy provided superior disease control compared with non-pemetrexed chemotherapy regimens, with PFS of 5.5 vs 3.0 months, respectively. In a retrospective analysis of 1882 patients with lung adenocarcinoma, 46 patients with EGFR exon20ins had similar OS on platinum-based chemotherapy to that of 258 patients with EGFR exon19 deletion/L858R mutation (26 vs 31 months, respectively; p = 0.53) [36]. This further demonstrates the unmet need for targeted agents in the exon20ins populations.
The activity of chemotherapy in patients with HER2 mutation is similar to that of patients with EGFR exon20ins. From the European EUHER2 cohort, the ORR and median PFS were 43.5% and 6 months (95% CI 5–7.1) in the first-line chemotherapy setting (n = 93) [24]. Most patients (n = 71) received first-line platinum-based doublet with a pemetrexed backbone. In the second-line setting, chemotherapy yielded an ORR of 10% and median PFS of 4.3 months (95% CI 3.1–5) (n = 52) [24]. In the second line, most patients received monotherapy with erlotinib (n = 15), docetaxel (n = 9), or pemetrexed (n = 7). Median OS was 24 (95% CI 10.1–36.4) and 19.4 (95% CI 9.6–24.7) with first- and second-line therapy, respectively.
4.2 Immune-Checkpoint Inhibitors
Patients with classic EGFR mutant NSCLC do not benefit from immune-checkpoint inhibitors (ICIs) [37, 38]. Additionally, combining EGFR TKIs and ICIs increases toxicity without evidence of clinical benefit [39]. In a study of 263 NSCLC with EGFR exon20ins, median tumor mutational burden was 3.6 mutations per megabase, similar to that of other EGFR mutant NSCLC (3.6 mutations per megabase; p = 0.31) and significantly lower than EGFR wild-type tumors (8.1 mutations per megabase; p < 0.0001) [40].
Although patients with exon20ins have largely been excluded from immunotherapy clinical trials, in retrospective analyses, patients with EGFR or HER2 exon20ins seem to derive greater benefit than patients with classic EGFR mutations [41,42,43]. In one retrospective analysis of 48 NSCLC patients treated with any ICI, Lau et al. showed that exon20ins were associated with better response (HER2 29%, n = 14; EGFR 50%, n = 6) than classic EGFR mutations, though these findings were not statistically significant (p = 0.07) [41]. Compared to classic mutations, exon20ins were associated with improved PFS (adjusted hazard ratio [HR] for HER2 0.35, p = 0.02; adjusted HR for EGFR 0.37, p = 0.10). Programmed death ligand 1 (PD-L1) expression was an independent prognostic factor for PFS (HR 0.42; 95% CI 0.23–0.76). ICIs were generally well tolerated, and in those patients who received subsequent TKI, no immune-related toxicity was observed, although the study was limited by its small sample size.
In one prospective series of 36 patients with EGFR exon20ins who received ICIs, the observed ORR was 25%, with PFS of 2.9 months, versus classical EGFR mutations, where ORR was 0% and PFS was 1.9 months [42]. The authors found that HER2 exon20ins had similar ORR and PFS to those of classic EGFR mutations (HR 1.1, p = 0.8) [35].
4.3 First- and Second-Generation EGFR TKIs
In contrast to other EGFR mutations in NSCLC, exon20ins are generally associated with resistance to first- and second-generation TKIs through steric hindrance of the drug binding pocket. The ORR ranged from 0 to 28%, with median PFS less than 4 months [34, 44, 45]. First-generation TKIs (gefitinib, erlotinib, and icotinib) bind to EGFR reversibly. The second-generation TKIs (afatinib, dacomitinib, and neratinib) bind irreversibly. In exon20ins, this mechanism is thought to require higher plasma concentrations for inhibition than is feasible in clinical practice, due to dose-limiting toxicities [19].
As a result, patients with EGFR exon20ins have better survival and response with chemotherapy compared to TKIs in the first line [45,46,47]. The OS of EGFR exon20ins in the first-line treatment setting ranges from 7.1 to 16.8 months with TKI [46, 47] versus 6.3–28 months on chemotherapy [46]. One study combining TKI and platinum chemotherapy found an OS of 16.4 months [45]. The ORR ranges from 0 to 8.7% with TKI [17, 46] versus 23–29% on chemotherapy [46, 48, 49].
In the later lines of therapy, the OS with TKI is approximately 12.9–15.3 months [47, 50], with one study also reporting 17.1 months with chemotherapy and 8.0 months with immunotherapy [47].
4.4 HER2 TKIs
In a randomized phase II trial, patients with HER2 mutant NSCLC received pan-HER TKI neratinib with or without mammalian target of rapamycin inhibitor temsirolimus [51] based on results from preclinical data [27] and a phase I trial of HER2 mutant solid tumors [52]. No responses were observed in the neratinib group, compared with an ORR of 21% in the neratinib plus temsirolimus arm [51]. Given the high prevalence of exon20ins in HER2 mutant NSCLC, most patients were expected to have this subtype.
5 Response to Novel TKIs
5.1 Mobocertinib (TAK-788)
Mobocertinib is a selective oral TKI targeting EGFR exon20ins. A phase I/II trial of 28 previously treated EGFR exon20ins patients found an ORR of 43% (95% CI 24–63), with median PFS of 7.3 months (95% CI 4.4–15.6) [53]. This group was compared to 71 matched real-world EGFR exon20ins patients who did not receive mobocertinib, and had an ORR of 13%, with a median PFS duration of 3.5 months. In 12 patients with brain metastases at baseline, ORR was 25%, compared to 56% (95% CI 30–80%) in patients without brain metastases [53]. The median PFS was 3.7 months (95% CI 1.8–15.9) versus 10.2 months (95% CI 5.6–NR) [53]. Grade 3 or higher treatment-related adverse events occurred in 40% of patients, the most common being diarrhea (21%). As a result, mobocertinib was granted Food and Drug Administration (FDA) Breakthrough Therapy designation in April 2020 [54].
Data from the phase II extension (EXCLAIM) cohort of 114 platinum-pretreated patients were presented at American Society of Clinical Oncology (ASCO) 2021. These findings confirmed an ORR of 28% (95% CI 20–37) and median PFS of 7.3 months (95% CI 5.5–9.2) [55]. As a result, mobocertinib was granted accelerated approval by the FDA for treatment of advanced-stage NSCLC harboring EGFR exon20ins following disease progression on platinum-based chemotherapy in September 2021 [56]. Table 1 shows the major efficacy and safety endpoints of mobocertinib and amivantamab. (Also see Table 2 for a comprehensive comparison of exon20ins agents.)
The phase III EXCLAIM-2 trial of mobocertinib versus platinum-based chemotherapy in advanced NSCLC with EGFR exon20ins in the first-line setting is currently ongoing (NCT04129502).
Preclinical data on mobocertinib in HER2 mutant NSCLC have shown promising results both in vitro and in mouse models with varying activity against certain variants [57], and although the EXCLAIM study originally included HER2 mutant patients, enrollment was halted for this arm.
5.2 Osimertinib
Osimertinib is an oral, potent, irreversible EGFR TKI selective for the founder EGFR mutation and the EGFR T790M resistance mutations. Although preclinical studies showed activity in EGFR exon20ins cell lines and xenografts [18, 58, 59], and in a retrospective analysis of six patients with EGFR exon20ins treated with osimertinib 80 mg daily, four patients achieved PR [60], with median PFS of 6.2 months (95% CI 5.0–12.9), since there is a significant overlap in terms of conformation between EGFR exon20ins and wild-type EGFR in the ATP binding pocket [4], osimertinib lacks selectivity against EGFR exon20ins.
In a phase I/II study of osimertinib in EGFR exon20ins, authors found median PFS of 3.8 months, with OS of 15.8 months [61]. The results from the phase II ECOG-ACRIN 5162 trial were presented at ASCO 2021. In 21 NSCLC patients with EGFR exon20ins treated with a “double dose (160 mg)” of osimertinib, the ORR was 25%, with median PFS of 9.7 months (95% CI 4.1–NR) [62]. Most common adverse events included anemia (9.5%), fatigue (9.5%), and QT interval prolongation (9.5%).
Although osimertinib demonstrates activity in wild-type HER2 overexpression, it has failed to show benefit in HER2 exon20ins [63].
5.3 Poziotinib
Poziotinib is an oral, irreversible, pan-HER TKI. It is more potent than afatinib and osimertinib in EGFR and HER2 exon20ins. Poziotinib resulted in an ORR of 43% among 44 patients with EGFR exon20ins in a phase II trial, with median PFS of 5.6 months (95% CI 5.06–NR) (NCT03066206) [64]. Within the HER2 exon20ins cohort, the ORR among 12 patients was 42%, with median PFS of 5.1 months. Grade 3 or higher treatment-related adverse events occurred in 56% of patients and were mostly rash and diarrhea.
The phase II ZENITH20 trial was initiated in an attempt to confirm these findings. Study patients in the EGFR exon20ins mutation cohort had at least one line of prior treatment, and demonstrated an ORR of 14.8% (95% CI 8.9–22.6) and median PFS of 4.2 months [64]. Within the HER2 exon20ins cohort, the ORR was 27.8% (95% CI 59.4–79.2), with median PFS of 5.5 months (95% CI 3.9–5.8) [65]. These results led to FDA fast-track designation of poziotinib for previously treated NSCLC with HER2 exon20ins in March 2021 [66]. Further analyses revealed decreased adverse effects with 8 mg twice daily rather than 16 mg daily dosing.
A single-center expanded access program of 30 patients with EGFR (n = 22) or HER2 (n = 8) exon20ins NSCLC on poziotinib resulted in an ORR of 23% in the EGFR cohort and 50% in the HER2 cohort [67]. The median PFS was 5.6 months (95% CI 3.6–6.7), and median OS was 9.5 months (95% CI 5.3–NR). In this program, 66% of patients had grade 3 or 4 toxicities. This confirms patients with exon20ins have variable response to poziotinib with significant toxicity. It has been proposed that this toxicity is due to potent inhibition of wild-type EGFR that is not selective for exon20ins [68].
5.4 Tarloxotinib
Tarloxotinib is a potent, irreversible pan-HER TKI [69]. Tarloxotinib is a prodrug that becomes the active metabolize tarloxotinib-E under hypoxic conditions, thus preferentially accumulating in hypoxic tumors relative to healthy tissue [70]. In preclinical models, tarloxotinib was effective in EGFR and HER2 exon20ins or fusions involving NRG1 encoding for neuregulin 1.
In the RAIN-701 trial (NCT03805841), patients with EGFR exon20ins or HER2-activating mutations received weekly tarloxotinib 150 mg/m2 intravenously. The ORR was 22% (2/9), with grade 3 adverse events including QTc prolongation (35%), rash (4.3%), diarrhea (4.3%), and elevated transaminase levels (4.3%) [71]. Tarloxotinib demonstrated clinical activity against HER2 but not EGFR exon20ins, leading to a recruitment interruption.
5.5 Pyrotinib
Pyrotinib is an irreversible HER1, HER2, and HER4 TKI with demonstrated activity in breast cancer [72]. In lung cancer xenograft models, pyrotinib showed superior activity compared to afatinib or trastuzumab emtansine [73]. In a phase II trial of advanced HER2-aberrant NSCLC previously treated with platinum-based chemotherapy, pyrotinib showed an ORR of 30%, with median PFS of 6.9 months and median OS of 14.4 months [74]. Grade 3–4 treatment-related adverse effects occurred in 28.3% of patients, including grade 3 diarrhea in 20% of participants.
Ongoing trials of pyrotinib include the phase II PEER20 EGFR or HER2 exon20ins (NCT04063462) and the randomized phase III PYRAMID-1 trial comparison with second-line pyrotinib versus docetaxel (NCT04447118). Another phase II trial combining pyrotinib with anti-PD-1 antibodies in patients with NSCLC harboring HER2 but not EGFR insertion mutations is also active at this time (NCT04144569).
6 Response to Novel Antibodies
6.1 Amivantamab
Amivantamab is a bispecific immunoglobulin G1 (IgG1) antibody targeting EGFR and mesenchymal–epithelial transition. Amivantamab was the first treatment to receive accelerated FDA approval for EGFR exon20ins. Its mechanism is through blocking ligands from binding to these receptors while also inducing antibody-dependent cytotoxicity [75, 76]. In the phase I CRYSALIS trial (NCT02609776), amivantamab is being studied as both a single agent, in combination with third-generation TKI lazertinib, and in combination with platinum-based chemotherapy. At interim analysis of 39 patients with EGFR exon20ins receiving single-agent amivantamab, the ORR was 40% (95% CI 29–51), with median PFS of 8.3 months (95% CI 6.5–10.9) [77], and led to FDA accelerated approval in this setting. In a sub-analysis of patients previously treated with platinum-based chemotherapy, the ORR was 41%, with median PFS of 8.6 months. The most common adverse events were rash (86%), infusion-related reactions (66%), and paronychia (45%), with grade 3 or higher adverse events in 6% of participants. Table 1 shows the major efficacy and safety endpoints of mobocertinib and amivantamab in comparison with TAS6417 /CLN-081.
6.2 Cetuximab
Cetuximab is an anti-EGFR monoclonal antibody that sterically hinders EGFR dimer formation [78]. Preclinical models show that mutant EGFR monomers have enhanced dimerization, supporting utilization of this agent [79]. In preclinical studies, cetuximab in combination with a TKI such as erlotinib, afatinib, or osimertinib showed activity against EGFR exon20ins [60, 79,80,81]. The combination of cetuximab with TKI is limited by its toxicity profile, with grade 3 or higher treatment-related adverse events occurring in approximately 70% of patients and a treatment discontinuation rate of 30% in the randomized phase II SWOG S1403 trial of cetuximab plus afatinib [82]. Similarly, in the phase II AFACET trial (NCT03727724), afatinib plus cetuximab resulted in an ORR of 47%, with median PFS of 5.5 months [83]. Treatment-related adverse events grade 3 or higher occurred in 59% of patients, namely rash (18%) and diarrhea (18%).
6.3 Trastuzumab Emtansine
Trastuzumab is an IgG1 monoclonal antibody that when conjugated to emtansine, an antimicrotubule agent, is used in breast cancer patients with HER2 amplification/overexpression [84]. In a phase II basket trial of HER2-altered cancers, the partial response rate of the NSCLC cohort was 44% (95% CI 22–69), with median PFS of 5 months (95% CI 3–9) [85]. Toxicities were largely grade 1–2 and included infusion-related reactions, elevation in transaminases, anemia, and thrombocytopenia.
In another phase II trial of trastuzumab emtansine, in 22 previously treated NSCLC HER2 exon20ins patients, the ORR was 38.1% (90% CI 23–55.9), with median PFS of 2.8 months (95% CI 1.4–4.4) [86]. The median OS was 8.1 months (95% CI 3.5–13.2). Grade 3 or higher toxicities included cardiac dysfunction (4.5%), anemia (4.5%), hypertension (4.5%), and brain hemorrhage (4.5%).
6.4 Trastuzumab Deruxtecan
Trastuzumab is another antibody–drug conjugate, which consists of trastuzumab conjugated to deruxtecan, a topoisomerase I inhibitor. In a phase I trial of HER2-mutant and/or HER2-expressing cancers, the NSCLC HER2 mutant cohort had an ORR of 72.7 (8/11), with median PFS of 11.3 months (95% CI 8.1–14.3) [87]. The most common treatment-related adverse events included gastrointestinal and hematological complications. Grade 3 or higher treatment-related adverse events occurred in 62.7% of patients, with the most common including anemia (25.4%), decreased neutrophil count (20.3%), decreased white blood cell count (18.6%), and decreased platelet count (15.3%).
The Destiny-Lung01 study (NCT03505710), presented at the ASCO meeting in 2020, gave trastuzumab deruxtecan a breakthrough therapy designation. The data were further updated with the HER2 mutant cohort of 91 patients at the European Society of Medical Oncology Congress in 2021, reporting an ORR of 55%, with median PFS of 9.3 months and median OS of 17.8 months [76]. Recently, trastuzumab deruxtecan gained FDA accelerated approval (in August 2022) and became the first antibody–drug conjugate to be approved in NSCLC. While the addition of HER2 targeted therapy in NSCLC was a long-awaited achievement, the toxicity profile of trastuzumab deruxtecan is notable: 88 of the 91 study patients experienced adverse events that were attributable to trastuzumab deruxtecan, while 42 of these events were grade 3 or higher. Two fatal adverse events were attributed to the study medication. The most notable attributed adverse events were nausea, neutropenia, and pneumonitis. In particular, drug-induced pneumonitis is of particular concern, with 26% for all grades and 6.6% for grade 3 and higher, respectively [76].
The Destiny-Lung02 phase II study is ongoing to compare efficacy and safety of two doses (6.4 mg/kg and 5.4 mg/kg) (NCT04644237). Trastuzumab deruxtecan is also being explored in the front-line setting of advanced/metastatic NSCLC in the Destiny-Lung04 trial (NCT05048797). Other ongoing studies are looking at trastuzumab deruxtecan in combination with pembrolizumab (NCT04042701), which in preclinical models has shown greater efficacy than either drug alone [88]. Trastuzumab deruxtecan is also being studied in combination with chemotherapy (NCT04686305).
6.5 Trastuzumab and Pertuzumab
Trastuzumab is often combined with another HER2-targeting monoclonal antibody, pertuzumab. In a phase II trial of previously treated NSCLC HER2 exon20ins, this combination resulted in an ORR of 29%, with median PFS of 6.8 months (95% CI 4.0–8.5) [89]. Grade 3 or 4 toxicities were seen in 64% of patients and included neutropenia (33%), diarrhea (13%), and anemia (9%), though none resulted in treatment discontinuation.
7 Future Compounds
7.1 TAS6417/CLN-081
TAS6417 (CLN-081) is an irreversible EGFR TKI with activity against both common mutations and exon20ins. This agent was engineered to fit inside the ATP binding pocket of EGFR exon20ins kinase, while sparing wild-type EGFR [20]. Preclinical studies have confirmed selectivity for EGFR exon20ins over wild-type EGFR. In xenograft models, TAS6417 inhibited EGFR phosphorylation to block PI3K-AKT and RAS-MAPK signaling pathways, ultimately causing tumor regression.
At interim analysis of a phase I/II trial in previously treated EGFR exon20ins (NCT04036682), presented at ASCO 2021, TAS6417 had an ORR of 40% (10/25) [90]. Grade 3 treatment-related adverse events included anemia (5%), diarrhea (3%), and alkaline phosphatase (3%). Updated data presented at ASCO 2022 showed an ORR of 38.4% and overall median PFS of 10 months (n = 73), with intracranial response demonstrated in a few patients. The toxicity profile also appears to be favorable (Table 1) [80]. Given the above data, TAS6417/CLN-081 has been granted breakthrough therapy designation by the FDA.
7.2 LNG-451 (BLU-451)
LNG-451 (BLU-451) is an oral, covalent inhibitor of EGFR exon20ins with CNS penetration. Given that 30% of NSCLC patients develop brain metastases, agents that cross the blood–brain barrier are important therapeutic options. Preclinical models show that BLU-4551 spares wild-type EGFR cells and has activity in the brain and spinal cord [91]. Further analysis showed that BLU-451 had similar potency to mobocertinib and greater potency than osimertinib in EGFR exon20ins [92]. A phase I/II clinical trial of BLU-451 in EGFR exon20ins is ongoing (NCT0521873).
7.3 DZD9008
DZD9008 is a novel, oral, irreversible EGFR and HER2 exon20ins variant-selective TKI. The WU-KONG1 trial (NCT03974022) is an ongoing phase I/II study assessing activity and safety of this drug. In 31 patients with EGFR exon20ins (phase II study presented at ASCO 2021), the ORR was 48.4% (15/31) [93]. The most common grade 3 adverse events were diarrhea (5%) and rash (1%).
7.4 BDTX-189
BDTX-189 is an oral, irreversible TKI with high selectivity in preclinical studies for HER2 and EGFR mutations over wild-type EGFR [94].
In the phase I/II MasterKey-01 study of patients with advanced EGFR, HER2, or HER3 mutations (NCT04209465), preliminary data from 46 patients, including five HER2 exon20ins and five EGFR exon20ins, were presented at the ASCO 2021 meeting [94]. The ORR was 7%, though neither patient with response had exon20ins. Grade 3 treatment-related adverse events included diarrhea (8%) and vomiting (3%). Ultimately, Black Diamond Therapeutics stopped development to focus on other therapeutics.
7.5 BDTX-1535
BDTX-1535 is a brain-penetrant EGFR inhibitor for the treatment of patients with glioblastoma and NSCLC patients with intrinsic or acquired resistance mutations. A phase I trial of this drug in NSCLC with uncommon EGFR mutations and acquired resistance EGFR mutations is currently enrolling.
7.6 Compound 1A
Compound 1A was structurally designed to bind the deep hydrophobic pocket at the back of the ATP binding site exposed when osimertinib binds wild-type EGFR [95]. This drug has a similar pyrimidine core structure that binds both EGFR Cys797 as well as the hydrophobic pocket. It has shown broad and potent activity against EGFR and HER2 exon20ins. In preclinical models of EGFR exon20ins, Compound 1A inhibited EGFR phosphorylation and cell proliferation with greater selectivity for mutant over wild-type EGFR than second-generation TKIs or poziotinib. Although early results are promising, clinical utility may be limited by low oral bioavailability and short half-life [96].
7.7 DS2087b
DS2087b is an oral, highly selective inhibitor of EGFR and HER2 exon20ins. In preclinical models, DS2087b was 15 times more potent in the inhibition of EGFR exon20ins cell line growth over wild-type EGFR [97]. The selectivity of this drug resembles that of poziotinib, so clinical trials are necessary to determine safety and tolerability.
7.8 JMT-101
JMT-101 is an IgG1 monoclonal antibody targeting EGFR. A phase Ib trial of JMT-101 combined with either afatinib or osimertinib in EGFR exon20ins is currently ongoing (NCT04448379).
7.9 BI 1810631
BI1810631 is an HER2 exon 20 inhibitor being developed by Boehringer Ingelheim, and a study in patients with solid tumors harboring HER2 aberrations is ongoing (NCT04886804).
8 Future Directions
As effective targeted therapeutics become more widely available, mechanisms of resistance will need to be further explored. Combinations of these therapies with other agents such as chemotherapy or immunotherapy may be able to prevent at least some of the resistance mechanisms; however, these should be studied in clinical trials with caution, while better toxicity management should be developed both in the single-agent and combinatory settings.
Additionally, these agents should be evaluated in different settings, such as the frontline setting, to assess their efficacy and safety against the current standard of care of platinum-based chemotherapy. Just as it was shown for the classic EGFR mutations, treatment with upfront targeted therapy may ultimately have survival benefit. Furthermore, the use of these agents for adjuvant therapy may be explored, although the toxicity profile would need to be further optimized for the adjuvant setting, where a proportion of patients may already be cured with surgical intervention (and/or chemotherapy) alone. The field of NSCLC is moving quickly. The neoadjuvant space may be where we would ultimately be able to better learn about the resistance mechanisms of these agents, as comparative analysis may be possible among those who were able to mount a pathological complete response versus those with a sizable amount of residual disease.
9 Conclusion
The recent approvals of mobocertinib and amivantamab for EGFR exon20ins as well as trastuzumab deruxtecan for HER2 exon20ins represent promising advances in treating these mutant cancers. However, these drugs have significant side effect profiles, especially diarrhea, nausea, and rash for mobocertinib and amivantamab, and chemotherapy-related adverse events as well as pneumonitis for trastuzumab deruxtecan. Further research needs to focus on mitigating these side effects so patients can have improved quality of life while on these medications. Further data on those patients who are likely to benefit from these agents as well as those who may be at a higher risk of adverse events are yet to be elucidated.
References
Lung Cancer Fact Sheet. 2022 [cited 2022 May 12]. https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/resource-library/lung-cancer-fact-sheet. p.MsoNormal, li.MsoNormal, div.MsoNormalAvailable from: font-size:11.0ptAvailable from: }a:link, span.MsoHyperlinkAvailable from: }a:visited, span.MsoHyperlinkFollowedAvailable from: font-size:11.0ptAvailable from: mso-ansi-font-size:11.0ptAvailable from: mso-bidi-font-size:11.0ptAvailable from: }div.WordSection1.
Cancer facts and figures. 2022 [cited 2022 May 12]. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html. Accessed 10 May 2022.
Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10(11):760–74.
Oxnard GR, Lo PC, Nishino M, Dahlberg SE, Lindeman NI, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8(2):179–84.
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–61.
Ramalingam SS, Vansteenkiste J, Planchard D, Chul Cho B, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50.
Herbst RS, Tsuboi M, John T, Grohe C, Majem M, Goldman JW, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J Clin Oncol. 2020;38(18_suppl):LBA5.
Smeltzer MP, Wynes MW, Lantuejoul S, Soo R, Ramalingam SS, Varella-Garcia M, et al. The International Association for the study of lung cancer global survey on molecular testing in lung cancer. J Thorac Oncol. 2020;15(9):1434–48.
Ren S, Wang J, Ying J, Mitsudomi T, Lee DH, Wang Z, et al. Consensus for HER2 alterations testing in non-small-cell lung cancer. ESMO Open. 2022;7(1): 100395.
Sheikine Y, Rangachari D, McDonald DC, Huberman MS, Folch ES, VanderLaan PA, et al. EGFR testing in advanced non-small-cell lung cancer, a mini-review. Clin Lung Cancer. 2016;17(6):483–92.
Lanman RB, Mortimer SA, Zill OA, Sebisanovic D, Lopez R, Blau S, et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell-free circulating tumor DNA. PLoS ONE. 2015;10(10): e0140712.
Non-Small Cell Lung Cancer. [cited 2022 May 12]. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450. Accessed 10 May 2022.
Han JY, Kim SH, Lee YS, Lee SY, Hwang JA, Kim JY, et al. Comparison of targeted next-generation sequencing with conventional sequencing for predicting the responsiveness to epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy in never-smokers with lung adenocarcinoma. Lung Cancer. 2014;85(2):161–7.
Lin HM, Yin Y, Crossland V, Wu Y, Ou SHI. EGFR testing patterns and detection of EGFR Exon 20 insertions in the United States. JTO Clin Res Rep. 2022;3(3): 100285.
Arcila ME, Nafa K, Chaft JE, Rekhtman N, Lau C, Rev BA, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013;12(2):220–9.
Faber AC, Corcoran RB, Ebi H, Sequist LV, Waltman BA, Chung E, et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov. 2011;1(4):352–65.
Yang M, Xu X, Cai J, Ning J, Wery JP, Li QX. NSCLC harboring EGFR exon-20 insertions after the regulatory C-helix of kinase domain responds poorly to known EGFR inhibitors. Int J Cancer. 2016;139(1):171–6.
Hirano T, Yasuda H, Tani T, Hamamoto J, Oashi A, Ishioka K, et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget. 2015;6(36):38789–803.
Yasuda H, Park E, Yun CH, Sng NJ, Lucena-Araujo AR, Yeo WL, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5(216):216ra177.
Hasako S, Terasaka M, Abe N, Uno T, Ohsawa H, Hashimoto A, et al. TAS6417, A novel EGFR inhibitor targeting exon 20 insertion mutations. Mol Cancer Ther. 2018;17(8):1648–58.
Arcila ME, Chaft JE, Nafa K, Roy-Chowdhuri S, Lau C, Zaidinski M, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res. 2012;18(18):4910–8.
Tomizawa K, Suda K, Onozato R, Kosaka T, Endoh H, Sekido Y, et al. Prognostic and predictive implications of HER2/ERBB2/neu gene mutations in lung cancers. Lung Cancer. 2011;74(1):139–44.
Mazières J, Peters S, Lepage B, Cortot AB, Barlesi F, Beau-Faller M, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol. 2013;31(16):1997–2003.
Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate J, Witsuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006.
Li BT, Ross DS, Aisner DL, Chaft JE, Hsu M, Kako SL, et al. HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol. 2016;11(3):414–9.
Network CGAR. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50.
Perera SA, Li D, Shimamura T, Raso MG, Ji H, Chen L, et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc Natl Acad Sci USA. 2009;106(2):474–9.
Zhao S, Fang W, Pan H, Yang Y, Liang Y, Yang L, et al. Conformational landscapes of HER2 exon 20 insertions explain their sensitivity to kinase inhibitors in lung adenocarcinoma. J Thorac Oncol. 2020;15(6):962–72.
Mazières J, Barlesi F, Filleron T, Besse B, Monnet I, Beau-Faller M, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol. 2016;27(2):281–6.
Shah MP, Aredo JV, Padda K, Ramchandran KJ, Wakelee HA, Das MS, et al. EGFR exon 20 insertion NSCLC and response to platinum-based chemotherapy. Clin Lung Cancer. 2022;23(2):e148–53.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–57.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8.
Yang G, Li J, Xu H, Yang Y, Yang L, Xu F, et al. EGFR exon 20 insertion mutations in Chinese advanced non-small cell lung cancer patients: molecular heterogeneity and treatment outcome from nationwide real-world study. Lung Cancer. 2020;145:186–94.
Xu CW, Wang WX, Wang D, Wang QM, Pu XX, Zhu YC, et al. Pemetrexed-based chemotherapy for non-small-cell lung cancer patients with EGFR exon 20 insertion mutation: a multicenter study. Transl Lung Cancer Res. 2020;9(5):1853–61.
Naidoo J, Sima CS, Rodriguez K, Busby N, Nafa K, Ladanyi M, et al. Epidermal growth factor receptor exon 20 insertions in advanced lung adenocarcinomas: clinical outcomes and response to erlotinib. Cancer. 2015;121(18):3212–20.
Addeo A, Passaro A, Malapelle U, Banna GL, Subbiah V, Friedlaender A. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat Rev. 2021;96: 102179.
Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol. 2017;12(2):403–7.
Creelan BC, Yeh TC, Kim SW, Nogami N, Kim DW, Chow LQM, et al. A Phase 1 study of gefitinib combined with durvalumab in EGFR TKI-naive patients with EGFR mutation-positive locally advanced/metastatic non-small-cell lung cancer. Br J Cancer. 2021;124(2):383–90.
Riess JW, Gandara DR, Frampton GM, Madison R, Peled N, Bufill JA, et al. Diverse EGFR exon 20 insertions and co-occurring molecular alterations identified by comprehensive genomic profiling of NSCLC. J Thorac Oncol. 2018;13(10):1560–8.
Lau SCM, Fares AF, Le LW, Mackay KM, Soberano S, Chan SW, et al. Subtypes of EGFR- and HER2-mutant metastatic NSCLC influence response to immune checkpoint inhibitors. Clin Lung Cancer. 2021;22(4):253–9.
Negrao MV, Reuben A, Robichaux JP, Le X, Nilsson MB, Wu CJ, et al. Association of EGFR and HER-2 exon 20 mutations with distinct patterns of response to immune checkpoint blockade in non-small cell lung cancer. J Clin Oncol. 2018;36(15_suppl):9052.
Guisier F, Dubos-Arvis C, Vinas F, Doubre H, Ricordel C, Ropert S, et al. Efficacy and safety of anti-PD-1 immunotherapy in patients with advanced NSCLC with BRAF, HER2, or MET mutations or RET translocation: GFPC 01-2018. J Thorac Oncol. 2020;15(4):628–36.
Beau-Faller M, Prim N, Ruppert AM, Nanni-Metellus I, Lacave R, Lacroix L, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol. 2014;25(1):126–31.
Cardona AF, Rojas L, Zatarain-Barron ZL, Freitas HC, Granados ST, Castillo O, et al. EGFR exon 20 insertion in lung adenocarcinomas among Hispanics (geno1.2-CLICaP). Lung Cancer. 2018;125:265–72.
Wu JY, Yu CJ, Shih JY. Effectiveness of treatments for advanced non-small-cell lung cancer with exon 20 insertion epidermal growth factor receptor mutations. Clin Lung Cancer. 2019;20(6):e620–30.
DerSarkissian M, Li S, Galaznik A, Bhak R, Bocharova I, Kulalert T, et al. HSR19-084: real-world treatment patterns and clinical outcomes in non-small cell lung cancer patients with EGFR exon 20 insertion mutations. J Natl Compr Cancer Netw J Natl Compr Canc Netw. 2019;17(3.5):HSR19-084.
Udagawa H, Matsumoto S, Ohe Y, Satouchi M, Furuya S, Kim YH, et al. OA07.03 clinical outcome of non-small cell lung cancer with EGFR/HER2 exon 20 insertions identified in the LC-SCRUM-Japan. J Thorac Oncol. 2019;14(10):S224.
Zhao C, Li X, Su C, Chen X, Ren S, Zhou C. P1.01–111 EGFR exon20 insertion patients treated with first-line chemotherapy in non-small cell lung cancer. J Thorac Oncol. 2018;13(10):S507.
Chen D, Song Z, Cheng G. Clinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutations. Onco Targets Ther. 2016;9:4181–6.
Besse B, Soria JC, Yao B, Kris M, Chao B, Cortot A, et al. Neratinib (N) with or without temsirolimus (Tem) in patients (Pts) with non-small cell lung cancer (Nsclc) carrying Her2 somatic mutations: an international randomized phase II study p. v1.
Gandhi L, Bahleda R, Tolaney SM, Kwak EL, Cleary JM, Pandya SS, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol. 2014;32(2):68–75.
Riely GJ, Neal JW, Camidge DR, Spira AI, Piotrowska Z, Costa DB, et al. Activity and safety of mobocertinib (TAK-788) in previously treated non-small cell lung cancer with. Cancer Discov. 2021;11(7):1688–99.
Takeda announces US FDA Breakthrough Therapy Designation for mobocertinib (TAK-788) for the treatment of NSCLC patients with EGFR exon 20 insertion mutations. April 27, 2020 [cited 2022 May 10]. https://www.takeda.com/newsroom/newsreleases/2020/takeda-announces-u.s.-fda-breakthrough-therapy-designation-for-mobocertinib-tak-788-for-the-treatment-of-nsclc-patients-with-egfr-exon-20-insertion-mutations/. Accessed 10 May 2022.
Ramalingam SS, Zhou C, Kim TM, Kim SW, Yang JCH, Riely GJ, et al. Mobocertinib (TAK-788) in EGFR exon 20 insertion (ex20ins)+ metastatic NSCLC (mNSCLC): Additional results from platinum-pretreated patients (pts) and EXCLAIM cohort of phase 1/2 study. J Clin Oncol. 2021;39(15_suppl):9014.
FDA grants accelerated approval to mobocertinib for metastatic non- small cell lung cancer with EGFR exon 20 insertion mutations. 2021 [cited 2022 May 10]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mobocertinib-metastatic-non-small-cell-lung-cancer-egfr-exon-20. Accessed 10 May 2022.
Han H, Li S, Chen T, Fitzgerald M, Liu S, Peng C, et al. Targeting HER2 exon 20 insertion-mutant lung adenocarcinoma with a novel tyrosine kinase inhibitor mobocertinib. Cancer Res. 2021;81(20):5311–24.
Masuzawa K, Yasuda H, Hamamoto J, Nukaga S, Hirano T, Kawada I, et al. Characterization of the efficacies of osimertinib and nazartinib against cells expressing clinically relevant epidermal growth factor receptor mutations. Oncotarget. 2017;8(62):105479–91.
Floc’h N, Martin MJ, Riess JW, Orme JP, Staniszewska AD, Menard L, et al. Antitumor activity of osimertinib, an irreversible mutant-selective EGFR tyrosine kinase inhibitor, in NSCLC harboring EGFR exon 20 insertions. Mol Cancer Ther. 2018;17(5):885–96.
Fang W, Huang Y, Hong S, Zhang Z, Wang M, Gan J, et al. EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer. 2019;19(1):595.
Yasuda H, Ichihara E, Sakakibara-Konishi J, Zenke Y, Takeuchi S, Morise M, et al. A phase I/II study of osimertinib in EGFR exon 20 insertion mutation-positive non-small cell lung cancer. Lung Cancer. 2021;162:140–6.
Piotrowska Z, Wang Y, Sequist LV, Ramalingam SS. ECOG-ACRIN 5162: a phase II study of osimertinib 160 mg in NSCLC with EGFR exon 20 insertions. J Clin Oncol. 2020;38(15_suppl):9513.
Liu S, Li S, Hai J, Wang X, Chen T, Quinn MM, et al. Targeting HER2 aberrations in non-small cell lung cancer with osimertinib. Clin Cancer Res. 2018;24(11):2594–604.
Le X, Goldman JW, Clarke JM, Tchekmedyian N, Piotrowska Z, Chu D, et al. Poziotinib shows activity and durability of responses in subgroups of previously treated EGFR exon 20 NSCLC patients. J Clin Oncol. 2020;38(15 suppl):9514.
Le X, Cornelissen R, Garassino M, Clarke JM, Tchekmedyian N, Goldman JW, et al. Poziotinib in non-small-cell lung cancer harboring HER2 exon 20 insertion mutations after prior therapies: ZENITH20-2 trial. J Clin Oncol. 2022;40(7):710–8.
FDA grants fast track designation to Spectrum Pharmaceuticals’ poziotinib [cited 2022 May 10]. https://www.businesswire.com/news/home/20210311005211/en/FDA-Grants-Fast-Track-Designation-to-Spectrum-Pharmaceuticals%E2%80%99-Poziotinib. Accessed 10 May 2022.
Prelaj A, Bottiglieri A, Proto C, Lo Russo G, Signorelli D, Ferrara R, et al. Poziotinib for EGFR and HER2 exon 20 insertion mutation in advanced NSCLC: results from the expanded access program. Eur J Cancer. 2021;149:235–48.
Friedlaender A, Subbiah V, Russo A, Banna GL, Malapelle U, Rolfo C, et al. EGFR and HER2 exon 20 insertions in solid tumours: from biology to treatment. Nat Rev Clin Oncol. 2022;19(1):51–69.
Estrada-Bernal A, Doak AE, Le AT, Zhu H, Chen N, Silva S, et al. Abstract A157: Antitumor activity of tarloxotinib, a hypoxia-activated EGFR TKI, in patient-derived lung cancer cell lines harboring EGFR exon 20 insertions. Mol Cancer Ther. 2018;17(1_Supplement):A157.
Estrada-Bernal A, Le AT, Doak AE, Tirunagaru VG, Silva S, Bull MR, et al. Tarloxotinib is a hypoxia-activated pan-HER kinase inhibitor active against a broad range of HER-family oncogenes. Clin Cancer Res. 2021;27(5):1463–75.
Liu SV, Villaruz LC, Lee VHF, Zhu VW, Baik CS, Sacher A, et al. LBA61 first analysis of RAIN-701: study of tarloxotinib in patients with non-small cell lung cancer (NSCLC) EGFR exon 20 insertion, HER2-activating mutations and other solid tumours with NRG1/ERBB gene fusions. Ann Oncol. 2020;31:S1189.
Chen Q, Ouyang D, Anwar M, Xie N, Wang S, Fan P, et al. Effectiveness and safety of pyrotinib, and association of biomarker with progression-free survival in patients with HER2-positive metastatic breast cancer: a real-world, multicentre analysis. Front Oncol. 2020;10:811.
Wang Y, Qin Z, Wang Q, Rivard C, Jiang T, Gao G, et al. Comparison the anti-tumor effect of pyrotinib, afatinb and T-DM1 in lung cancer organoids and PDX models with HER2 mutation. J Clin Oncol. 2018;36(15 suppl):e24281.
Zhou C, Li X, Wang Q, Gao G, Zhang Y, Chen J, et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, phase II study. J Clin Oncol. 2020;38(24):2753–61.
Yun J, Lee SH, Kim SY, Jeong SY, Kim JH, Pyo KH, et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody. Diverse Models Cancer Discov. 2020;10(8):1194–209.
Moores SL, Chiu ML, Bushey BS, Chevalier K, Luistro L, Dorn K, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2016;76(13):3942–53.
Park K, John T, Kim SW, Lee JS, Shu CA, Kim DW, et al. Amivantamab (JNJ-61186372), an anti-EGFR-MET bispecific antibody, in patients with EGFR exon 20 insertion (exon20ins)-mutated non-small cell lung cancer (NSCLC). J Clin Oncol. 2020;38(15_suppl):9512.
Brand TM, Iida M, Wheeler DL. Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer Biol Ther. 2011;11(9):777–92.
Tsigelny IF, Wheler JJ, Greenberg JP, Kouznetsova VL, Stewart DJ, Bazhenova L, et al. Molecular determinants of drug-specific sensitivity for epidermal growth factor receptor (EGFR) exon 19 and 20 mutants in non-small cell lung cancer. Oncotarget. 2015;6(8):6029–39.
Wheler JJ, Tsimberidou AM, Falchook GS, Zinner RG, Hong DS, Fok JY, et al. Combining erlotinib and cetuximab is associated with activity in patients with non-small cell lung cancer (including squamous cell carcinomas) and wild-type EGFR or resistant mutations. Mol Cancer Ther. 2013;12(10):2167–75.
Hasegawa H, Yasuda H, Hamamoto J, Masuzawa K, Tani T, Nukaga S, et al. Efficacy of afatinib or osimertinib plus cetuximab combination therapy for non-small-cell lung cancer with EGFR exon 20 insertion mutations. Lung Cancer. 2019;127:146–52.
Goldberg SB, Redman MW, Lilenbaum R, Politi K, Stinchcombe TE, Horn L, et al. Randomized trial of afatinib plus cetuximab versus afatinib alone for first-line treatment of. J Clin Oncol. 2020;38(34):4076–85.
van Veggel B, van der Wekken AJ, Paats M, Hashemi SMS, Hendriks L, Sikorska K, et al. Interim results of a phase II single arm trial combining afatinib with cetuximab in patients with EGFRex20ins positive NSCLC. J Clin Oncol. 2021;39:9112.
Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91.
Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018;36(24):2532–7.
Iwama E, Zenke Y, Sugawara S, Daga H, Morise M, Yanagitani N, et al. Trastuzumab emtansine for patients with non-small cell lung cancer positive for human epidermal growth factor receptor 2 exon-20 insertion mutations. Eur J Cancer. 2022;162:99–106.
Tsurutani J, Iwata H, Krop I, Janne PA, Doi T, Takahashi S, et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020;10(5):688–701.
Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. 2018;17(7):1494–503.
Mazieres J, Lafitte C, Ricordel C, Greillier L, Negre E, Zalcman G, et al. Combination of trastuzumab, pertuzumab, and docetaxel in patients with advanced non-small-cell lung cancer harboring HER2 mutations: results from the IFCT-1703 R2D2 trial. J Clin Oncol. 2022;40(7):719–28.
Piotrowska Z, Yu HA, Yang JCH, Koczywas M, Smit EF, Tan DSW, et al. Safety and activity of CLN-081 (TAS6417) in NSCLC with EGFR Exon 20 insertion mutations (Ins20). J Clin Oncol. 2021;39(15_suppl):9077.
Pearson PG, Pandey A, Roth B, Saxton T, Estes DJ, Trivedi R, et al. LNG-451 (BLU-451), a potent inhibitor of EGFR exon 20 insertion mutations with high CNS exposure. In AACR. 2022. New Orleans.
Murray BW, Pandey A, Roth B, Saxton T, Estes DJ, Trivedi R, et al. LNG-451 (BLU-451) is a potent, CNS-penetrant, wild-type EGFR sparing inhibitor of EGFR exon 20 insertion mutations. In AACR. 2022. New Orleans.
Yang JCH, Wang M, Mitchell P, Fang J, Nian W, Chiu CH, et al. Preliminary safety and efficacy results from phase 1 studies of DZD9008 in NSCLC patients with EGFR Exon20 insertion mutations. J Clin Oncol. 2021;39(15_suppl):9008.
Schram AM, Ahnert JR, Patel MR, Jauhari S, Sachdev JC, Zhu VW, et al. Safety and preliminary efficacy from the phase 1 portion of MasterKey-01: A First-in-human dose-escalation study to determine the recommended phase 2 dose (RP2D), pharmacokinetics (PK) and preliminary antitumor activity of BDTX-189, an inhibitor of allosteric ErbB mutations, in patients (pts) with advanced solid malignancies. J Clin Oncol. 2021;39(15 suppl):3086.
Jang J, Son J, Park E, Kosaka T, Saxon JA, DeClercq DJH, et al. Discovery of a highly potent and broadly effective epidermal growth factor receptor and HER2 exon 20 insertion mutant inhibitor. Angew Chem Int Ed Engl. 2018;57(36):11629–33.
Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct Target Ther. 2019;4:5.
Nagamoto Y, Miyamoto M, Togashi N, Taira T, Jimbo T, Isoyama T, et al. Preclinical evaluation of DS-2087b, a novel and selective inhibitor of EGFR/HER2 exon 20 insertions. In ESMO virtual congress. 2020. Annals of oncology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Competing interests
The authors declare that they have no direct competing interests. Misako Nagasaka has received honorarium from Astra Zeneca (ad-board), Caris Life Sciences (consultant), Lilly (consultant), Daiichi Sankyo (ad-board), Takeda (speaker), Novartis (ad-board), EMD Serono (ad-board), Blueprint Medicines (speaker), Janssen (ad-board), Pfizer (ad-board), Genentech (ad-board), and Mirati (ad-board) and has received travel support from AnHeart Therapeutics. Other authors have no potential conflicts of interest to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Authors’ contributions
DB provided clinical interpretation and drafted and reviewed all versions of the manuscript. GK drafted and reviewed all versions of the manuscript. MN provided clinical interpretation and drafted and reviewed all versions of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Brazel, D., Kroening, G. & Nagasaka, M. Non-small Cell Lung Cancer with EGFR or HER2 Exon 20 Insertion Mutations: Diagnosis and Treatment Options. BioDrugs 36, 717–729 (2022). https://doi.org/10.1007/s40259-022-00556-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-022-00556-4