Abstract
The efficacy of pembrolizumab in the treatment-naïve non-small-cell lung cancer (NSCLC) patients was proved in the KEYNOTE-024 randomized trial. The aim of this systematic literature review was to identify and summarize the real world evidence (RWE) of overall survival (OS) in previously untreated patients with NSCLC receiving pembrolizumab monotherapy. A systematic search was conducted in PubMed (MEDLINE®) and EMBASE databases. Analyses were focused on survival data (median OS and survival rates at specific time points). To explore the population comparable with the KEYNOTE-024 study, we focused on studies enrolling at least 50% of patients at stage IV of cancer and ECOG performance status 0–2. A total of 41 RWE studies covering over 7600 advanced NSCLC patients naïve to systemic treatment were identified. Overall, survival outcomes reported in those studies vary considerably (median OS range: 3.0–34.6 months). Most RWE studies reported median OS shorter to that reported in KEYNOTE-024 (26.3 months), but about half of reported OS medians were in range of 95% confidence interval for OS as reported in KEYNOTE-024 trial (18.3–40.4 months). Patients with similar characteristics of stage and performance status to those of KEYNOTE-024 trial benefited the same with pembrolizumab monotherapy as their survival outcomes (18.9–22.8 months) were consistent with those reported in the clinical trial. RWE data showed substantially worse outcomes in patients with ECOG-PS 2+ compared to ECOG-PS 0–1 patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Primary lung cancer is the 2nd most common malignancy after breast cancer, and leading cause of deaths due to malignancy worldwide. Lung cancer accounted for approximately 1.8 million deaths in 2020 year [1]. Non-small-cell lung carcinoma (NSCLC) accounts for 80%–90% of lung cancers, and most patients with NSCLC present with advanced-stage unresectable disease (stage IIIB to IV) [2]. Therefore, patients with NSCLC are at great need for effective and safe systemic therapy that can prolong their life and improve its quality. Until recently, lung cancers were considered poorly immunogenic i.e. minimal benefit have been seen in studies of vaccines or cytokine modulation [3]. However, monoclonal antibodies directed against the immune-checkpoint molecules, such as programmed cell death 1 (PD-1) receptor or its ligand (PD-L1) significantly improved NSCLC therapy outcomes. Currently, patients with locally advanced and unresectable or metastatic NSCLC with no activating genetic abnormalities (EGFR, ALK or ROS1) should be offered immunotherapy as monotherapy or combined with chemotherapy as a standard approach [2].
Pembrolizumab is a humanized monoclonal antibody against PD-1 that has increased activity in tumours which express PD-L1. Pembrolizumab is approved as monotherapy for the first-line treatment of metastatic NSCLC in adults with PD-L1 expression on at least 50% of tumour cells with no EGFR mutation or ALK fusion [4]. Efficacy of pembrolizumab in the first-line treatment of patients with metastatic NSCLC and high PD-L1 expression was assessed in a randomised multicentre, open-label, controlled KEYNOTE-024 study. Patients were randomised (1:1) to receive pembrolizumab at a dose of 200 mg every 3 weeks (n = 154) or investigator’s choice platinum-containing chemotherapy (n = 151). Among the 154 patients treated with pembrolizumab in KEYNOTE-024 (median age 64.5 years), 59.7% were male and 35.1% and 64.9% of patients had ECOG performance status 0 and 1, respectively. The majority of patients (81.2%) had non-squamous-cell carcinoma (squamous-cell carcinoma in 18.8%). Brain metastases were present at 11.7% of patients. At 5 year follow-up (median time from randomization 59.9 months), 103 patients (66.9%) in the pembrolizumab group have died. Median overall survival (OS) was 26.3 months (95% CI 18.3 to 40.4 months) and Kaplan–Meier estimate of OS at 5 years was 31.9% [5]. Therefore, pembrolizumab as monotherapy has shown durable efficacy regarding OS for the first-line treatment of metastatic NSCLC in adults whose tumours express PD-L1 with a ≥ 50% tumour proportion score (TPS) under controlled clinical trial. However, it is also important to analyse the real world effectiveness beyond the strictly controlled environment of clinical trial, since those studies provides data for broader populations and includes patients typically excluded or underrepresented in clinical trials.
2 Purpose of the analysis
The aim of this systematic literature review was to identify and summarize the real world evidence (RWE) of OS in previously untreated patients with NSCLC with high PD-L1 status receiving pembrolizumab monotherapy.
3 Methods
A systematic review of observational studies on pembrolizumab monotherapy in previously untreated NSCLC patients was performed. Systematic search was conducted in PubMed (MEDLINE®) and EMBASE databases. The search strategy included both Text Words and MeSH terms for NSCLC and pembrolizumab, coupled with queries about actual study designs regarding real world evidence and corresponding synonyms. Search strategies are presented in Tables 1 and 2 (Supplementary materials). The cut-off date was 17th June 2022. No geographic restrictions were imposed, however, the search was limited to studies published in English. Only full-text publications were reviewed, abstracts and other conference reports were excluded.
The studies were selected independently by three researchers (N.W.; K.G.; M.D.). All studies were assessed according to the eligibility criteria:
-
1.
RWE studies—publications with the hallmarks of clinical trials, such as sample size determination, randomization etc., were excluded;
-
2.
use of pembrolizumab as monotherapy in first-line treatment—studies in previously treated patients as well as those in which pembrolizumab was administered as part of a combination therapy were excluded;
-
3.
presence of PD-L1 expression with a tumour proportion score (TPS) ≥ 50%;
-
4.
performance status according to the Eastern Cooperative Oncology Group (ECOG) scale ≤ 2;
-
5.
presence of distant metastases reported explicitly in the characteristics of the population of a given study or determined by the stage of the disease i.e. stage IV (studies in which patients with stage IV represented at least 50% of study population were allowed);
-
6.
presented data for OS or survival rates.
We developed a standardised data extraction form in MS Excel. Key data were extracted from all studies that met the inclusion criteria for the review, including study design, patient characteristics at baseline, and efficacy endpoints (both median OS and survival rates at individual time points). The data extraction was independently verified and validated; any discrepancies between reviewers were resolved through discussion or consultation with a third reviewer if necessary.
Analyses were focused on survival data (median OS and survival rates at specific time points). Although KEYNOTE-024 trial included only stage IV and ECOG-PS 0–1, we decided to include data from studies in which at least 50% patients had IV stage of cancer and ECOG performance status 0–2. Additional explorative analyses covered data from studies which reported separately ECOG-PS 0–1 and ECOG-PS 2 patients. Wherever possible, data for population similar to KEYNOTE-024 trial (i.e. only patients at IV stage of disease and with ECOG-PS 0–1) were extracted. Correlation between median OS and ECOG was explored.
Forest plots were generated to summarize median OS for the overall study populations and subpopulation groups of interest. We did not pool estimates of median OS or survival rates since meta-analysis methods for median survival ratio are not appropriate [6]. Data are summarized with median (range: min.–max.) statistics. For correlation of median OS and ECOG, brain or liver metastasis status or disease stage Spearman’s correlation coefficient were used [7]. We assumed correlation > 0.8 to be very strong, 0.6–0.8 to be moderately strong, 0.3–0.5 to be fair and < 0.3 to be poor [8]. Hazard ratio (HR) for OS data in predefined populations recognized by ECOG, brain or liver metastasis status or cancer stage were pooled with fixed effect inverse variance approach in Review Manager (RevMan), Version 5.4.1, The Cochrane Collaboration, 2020.
4 Results
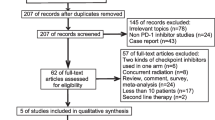
Through search in PubMed and EMBASE, we identified 825 publications and based on title and abstract we selected 112 potential studies (Supplementary materials, Fig. 1). After full-text review, we identified 41 RWE studies covering 46 cohorts of advanced NSCLC patients naïve to systemic treatment (Table 1). Most patients included in those studies had ECOG-PS 0–1 (median 81.6%), were at the IV stage of the disease (median 85.8%). A minority of patients presented squamous-cell carcinoma (median 21.7%). Other types of NSCLC were reported inconsistently. In 13 studies, all populations were at the IV stage of the disease, and in 6 studies, all patients had ESOG 0–1. Only 3 studies [9, 18, 48—EHR cohort] reported OS in populations similar to KEYNOTE-024 i.e. all patients at IV stage of the disease and with ECOG-PS 0–1. Median age of patients treated with pembrolizumab was 69 years. Most patients were male (median = 65.7%), and current or ex-smokers (median = 90.0%). Almost fifth of all patients had brain metastases (median = 19.3%) and 13.0% had liver metastases. Most of patients with brain metastases (median = 68.8%) had undergone previous local therapy (surgery or radiotherapy). Table 2 summarize patients’ characteristics in studies included in this review.
Median survival times varied across studies with 3.0 months minimum and 34.6 months maximum. Most RWE studies reported median OS shorter to that reported in KEYNOTE-024; however, about half of the reported median OS were within the 95% confidence interval as reported in KN-024 trial (18.3–40.4 months; Fig. 1). Expectedly, moderately strong negative correlation was seen between percentage of ECOG-PS2+ patients and OS (Spearman correlation coefficient rs = − 0.62).
When comparing OS for subpopulation with ECOG-PS 0–1 to subpopulation with ECOG-PS 2+ significant difference in favour of patients with better performance status was shown (median range: 14.3–28.9 months vs 1.5–12.8 months)—see Fig. 2A and B. Pooled HR for OS data showed significant difference in favour of ECOG-PS 0–1 population (HR = 0.35; 95%CI: 0.31, 0.38; p < 0.001)—see Fig. 3. In 3 studies of population analogous to KEYNOTE-024 (i.e. only patients at stage IV and ECOG-PS 0–1) median OS months were 22.8, 20.3 and 18.9 [9, 18, 48—EHR cohort, respectively]. As presented on Fig. 2A most results in ECOG-PS 0–1 population were within 95% confidence interval for OS reported in KEYNOTE-024 trial, while in ECOG-PS 2+ population none of OS reached 95% CI range from the clinical trial (Fig. 2B).
The 1-year and 2-years OS rates were achieved in 57.0% (median; range: 21.4%–92.0%) and 42.5% (median; range: 8.0%–79.0%)—see Table 3. Those values are lower to those reported in KEYNOTE-024 trial (70.3% and 54.8%, respectively).
Pooled HR for OS data showed significant difference in favour of females (HR = 1.15; 95%CI 1.03, 1.28; p = 0.01) and suggested a trend toward less benefit in never smokers; patients without brain metastases (HR = 1.21; 95%CI 1.06, 1.21; p = 0.004) as well as with no evidence of liver metastases (HR = 1.56; 95%CI 1.33, 1.84; p < 0.001) have significantly better prognosis. See Fig. 4A–D, respectively.
5 Discussion
We identified substantial number of RWE studies covering over 7600 previously untreated patients with metastatic NSCLC receiving pembrolizumab monotherapy. In general, the OS results vary across all analysed studies and we believe the differences are due to high heterogeneity of population included in each study in terms of known prognostic factors (i.e. ECOG-PS, tumor stage) as well as local practice on supportive management. Approximately half of reported OS medians were in range of 95% CI OS data form KEYNOTE-024 trial. Those observations are not surprising as KEYNOTE-024 trial covered only ECOG-PS 0–1 patients, and we have shown that ECOG-PS is an important factor affecting outcome. Similar observation was reported previously for pembrolizumab in pre-treated patients with NSCLC [51]. Also studies for other immune checkpoint inhibitors used for advanced NSCLC showed that patients with impaired performance status had significantly shorter survival compared to those with better performance status [52].
It’s worth to mention that KEYNOTE-024 trial covered only stage IV patients, while in RWE studies patients at stage III were also included. However percentage of patients with brain metastasis were overrepresented in real world data (19.6%) compared to KEYNOTE-024 trial (11.7% in pembrolizumab arm), and contrary to KEYNOTE-024 trial in real world settings not all patients received local treatment for brain metastasis. As expected, pooled HR data showed patients with brain or liver metastasis are at higher risk of death compared to patients without brain metastasis.
Review of RWE literature has shown that in real practice pembrolizumab monotherapy in patients with high PD-L1 expression may produce almost the same survival results as reported in the KEYNOTE-024 trial provided they have comparable stage and performance status. Although ESMO guidelines claim systemic therapy should be offered to all stage IV patients with PS 0–2, RWE data showed substantially worse outcomes in patients with ECOG-PS ≥ 2 compared to ECOG-PS 0–1 patients. In fact, in some countries (i.e. Poland) the use of immunotherapy is limited only to patients with ECOG-PS 0–1, what is in line with KEYNOTE-024 trial inclusion criteria. However, PePS2 trial, the only prospective phase 2 study that evaluated pembrolizumab monotherapy in patients with NSCLC and ECOG-PS 2, reported median OS of 9.8 (95%CI 7.1–14.6) months—values higher to those observed in the majority of ECOG-PS 2 RWE studies included in this review (1.5–12.8 months) [53]. On the other hand, RWE showed patients without distant metastases may also benefit from pembrolizumab monotherapy, but this findings has to be verify in controlled clinical study. Although a meta-analysis of clinical trials showed that pembrolizumab significantly improved overall survival in male individuals regardless of treatment line and regimen, we observed better outcomes in females [54].
Our secondary analysis has limitations, which result mainly from non-randomized settings and corresponding selection bias and many confounding variables we were unable to control for, even though the study focused on advanced NSCLC patients naïve to systemic treatment with similar characteristics of stage and performance status to those of KEYNOTE-024 trial. Many variables reflecting not only different patients’ characteristics, but also clinical practice arrangements, contribute to heterogeneity and affect the generalizability of the study results. Moreover, most likely, everyday clinical practice and locally arranged patients' access to treatment not harmonized with predefined study protocol most likely could substantially affect generalizability. We focused on mortality to restrain missing data and measurement bias, but anticipated high heterogeneity, which could not be further limited with no access to individual patients data but only aggregated statistics reported in individual studies. For the same reason, our analysis focused mainly on median overall survival as most consistently reported; the only pooled statistic we could reliably provide through meta-analysis, with low heterogeneity, was the overall survival hazard ratio (HR). Certainly, general treatment efficacy claims are unjustified and were not intended as the study explored real-world outcomes in uncontrolled and pragmatic settings.
We believe more RWE in the population close to that of the KEYNOTE-024 trial and specific subgroups should be collected to credibly explore pembrolizumab efficacy in real-world practice. It should not restrain from well-designed controlled trials to confirm pembrolizumab efficacy in new specific populations or clinical settings.
In conclusion, RWE studies of over 7600 advanced NSCLC patients new to systemic treatment displayed considerable variability in survival outcomes. While most studies reported a median OS shorter than that seen in the KEYNOTE-024 trial, patients with similar stage and performance status benefited equally from pembrolizumab monotherapy, with survival outcomes consistent with the clinical trial findings.
Data availability
Publicly available data.
Code availability
Not applicable.
References
World Health Organization. Cancer, key facts. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 22 Sept 2022.
Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192-iv237. https://doi.org/10.1093/annonc/mdy275. Erratum in: Ann Oncol. 2019;30(5):863–870, https://www.esmo.org/content/download/347819/6934778/1/ESMO-CPG-mNSCLC-15SEPT2020.pdf. Accessed 22 Sept 2022.
Raez LE, Fein S, Podack ER. Lung cancer immunotherapy. Clin Med Res. 2005;3(4):221–8. https://doi.org/10.3121/cmr.3.4.221.
https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf. Accessed 22 Sept 2022.
Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39(21):2339–49. https://doi.org/10.1200/JCO.21.00174.
Michiels S, Piedbois P, Burdett S, et al. Meta-analysis when only the median survival times are known: a comparison with individual patient data results. Int J Technol Assess Health Care. 2005;21(1):119–25. https://doi.org/10.1017/s0266462305050154.
Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71.
Chan YH. Biostatistics 104: correlational analysis. Singap Med J. 2003;44(12):614–9.
Alessi JV, Ricciuti B, Jiménez-Aguilar E, et al. Outcomes to first-line pembrolizumab in patients with PD-L1-high (≥50%) non-small cell lung cancer and a poor performance status. J Immunother Cancer. 2020;8(2): e001007. https://doi.org/10.1136/jitc-2020-001007.
Amrane K, Geier M, Corre R, et al. First-line pembrolizumab for non-small cell lung cancer patients with PD-L1 ≥50% in a multicenter real-life cohort: the PEMBREIZH study. Cancer Med. 2020;9(7):2309–16. https://doi.org/10.1002/cam4.2806.
Baldessari C, Pecchi A, Marcheselli R, et al. Body composition and inflammation impact in non-small-cell lung cancer patients treated by first-line immunotherapy. Immunotherapy. 2021;13(18):1501–19. https://doi.org/10.2217/imt-2021-0038.
Banna GL, Signorelli D, Metro G, et al. Neutrophil-to-lymphocyte ratio in combination with PD-L1 or lactate dehydrogenase as biomarkers for high PD-L1 non-small cell lung cancer treated with first-line pembrolizumab. Transl Lung Cancer Res. 2020;9(4):1533–42. https://doi.org/10.21037/tlcr-19-583.PMID:32953525;PMCID:PMC7481583.
Banna GL, Cantale O, Friedlaender A, et al. Risk of SARS-CoV2-related mortality in non-small cell lung cancer patients treated with first-line immunotherapy alone or in combination with chemotherapy. Cancer Invest. 2022;40(5):406–12. https://doi.org/10.1080/07357907.2021.1970761.
Banna GL, Tiseo M, Cortinovis DL, et al. Host immune-inflammatory markers to unravel the heterogeneous outcome and assessment of patients with PD-L1 ≥50% metastatic non-small cell lung cancer and poor performance status receiving first-line immunotherapy. Thorac Cancer. 2022;13(3):483–8. https://doi.org/10.1111/1759-7714.14256.
Bureau M, Chatellier T, Perennec T, et al. Baseline tumour size is an independent prognostic factor for overall survival in PD-L1 ≥ 50% non-small cell lung cancer patients treated with first-line pembrolizumab. Cancer Immunol Immunother. 2022;71(7):1747–56.
Cavaille F, Peretti M, Garcia ME, et al. Real-world efficacy and safety of pembrolizumab in patients with non-small cell lung cancer: a retrospective observational study. Tumori. 2021;107(1):32–8.
Chen Y, Wang Y, Yang Z, et al. Pembrolizumab alone or combined with chemotherapy in advanced NSCLC With PD-L1 ≥50%: results of a retrospective study. Front Oncol. 2021;11: 691519. https://doi.org/10.3389/fonc.2021.691519.
Cortellini A, Tiseo M, Banna GL, et al. Clinicopathologic correlates of first-line pembrolizumab effectiveness in patients with advanced NSCLC and a PD-L1 expression of ≥ 50. Cancer Immunol Immunother. 2020;69(11):2209–21. https://doi.org/10.1007/s00262-020-02613-9.
Cramer Welle CM, Verschueren MV, Tonn M, et al. Real-world outcomes versus clinical trial results of immunotherapy in stage IV non-small cell lung cancer (NSCLC) in the Netherlands. Sci Rep. 2021;11(1):6306. https://doi.org/10.1038/s41598-021-85696-3.
Dall’Olio FG, Calabrò D, Conci N, Argalia G, et al. Baseline total metabolic tumour volume on 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography-computed tomography as a promising biomarker in patients with advanced non-small cell lung cancer treated with first-line pembrolizumab. Eur J Cancer. 2021;150:99–107. https://doi.org/10.1016/j.ejca.2021.03.020. (Epub 2021 Apr 20).
Dudnik E, Moskovitz M, Rottenberg Y, et al. Pembrolizumab as a monotherapy or in combination with platinum-based chemotherapy in advanced non-small cell lung cancer with PD-L1 tumor proportion score (TPS) ≥50%: real-world data. Oncoimmunology. 2021;10(1):1865653. https://doi.org/10.1080/2162402X.2020.1865653.
Facchinetti F, Mazzaschi G, Barbieri F, et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur J Cancer. 2020;130:155–67. https://doi.org/10.1016/j.ejca.2020.02.023.
Friedlaender A, Metro G, Signorelli D, et al. Impact of performance status on non-small-cell lung cancer patients with a PD-L1 tumour proportion score ≥50% treated with front-line pembrolizumab. Acta Oncol. 2020;59(9):1058–63. https://doi.org/10.1080/0284186X.2020.1781249.
Frost N, Kollmeier J, Vollbrecht C, et al. KRASG12C/TP53 co-mutations identify long-term responders to first line palliative treatment with pembrolizumab monotherapy in PD-L1 high (≥50%) lung adenocarcinoma. Transl Lung Cancer Res. 2021;10(2):737–52. https://doi.org/10.21037/tlcr-20-958.
Frost N, Kollmeier J, Misch D, et al. Pembrolizumab as first-line palliative therapy in PD-L1 overexpressing (≥ 50%) NSCLC: real-world results with special focus on PS ≥ 2, brain metastases, and steroids. Clin Lung Cancer. 2021;22(5):411–22. https://doi.org/10.1016/j.cllc.2021.02.001.
Jiménez Galán R, Prado-Mel E, Pérez-Moreno MA, et al. Influence of performance status on the effectiveness of pembrolizumab monotherapy in first-line for advanced non-small-cell lung cancer: results in a real-world population. Biology (Basel). 2021;10(9):890. https://doi.org/10.3390/biology10090890.
Geiger-Gritsch S, Olschewski H, Kocher F, et al. Real-world experience with anti-PD-1/PD-L1 monotherapy in patients with non-small cell lung cancer: a retrospective Austrian multicenter study. Wien Klin Wochenschr. 2021;133(21–22):1122–30. https://doi.org/10.1007/s00508-021-01940-w.
Grosjean HAI, Dolter S, Meyers DE, et al. Effectiveness and safety of first-line pembrolizumab in older adults with PD-L1 positive non-small cell lung cancer: a retrospective cohort study of the alberta immunotherapy database. Curr Oncol. 2021;28(5):4213–22. https://doi.org/10.3390/curroncol28050357.
Hasegawa T, Yanagitani N, Utsumi H, et al. Association of high neutrophil-to-lymphocyte ratio with poor outcomes of pembrolizumab therapy in high-PD-L1-expressing non-small cell lung cancer. Anticancer Res. 2019;39(12):6851–7. https://doi.org/10.21873/anticanres.13902.
Holtzman L, Moskovitz M, Urban D, et al. dNLR-based score predicting overall survival benefit for the addition of platinum-based chemotherapy to pembrolizumab in advanced NSCLC with PD-L1 tumor proportion score ≥50. Clin Lung Cancer. 2022;23(2):122–34. https://doi.org/10.1016/j.cllc.2021.12.006.
Hosoya K, Fujimoto D, Morimoto T, et al. Clinical factors associated with shorter durable response, and patterns of acquired resistance to first-line pembrolizumab monotherapy in PD-L1-positive non-small-cell lung cancer patients: a retrospective multicenter study. BMC Cancer. 2021;21(1):346.
Ikezawa Y, Mizugaki H, Morita R, et al. Current status of first-line treatment with pembrolizumab for non-small-cell lung cancer with high PD-L1 expression. Cancer Sci. 2022;113(6):2109–17. https://doi.org/10.1111/cas.15361.
Imai H, Kishikawa T, Minemura H, et al. Pretreatment glasgow prognostic score predicts survival among patients with high PD-L1 expression administered first-line pembrolizumab monotherapy for non-small cell lung cancer. Cancer Med. 2021;10(20):6971–84. https://doi.org/10.1002/cam4.4220.
Isono T, Kagiyama N, Shibata S, et al. A retrospective analysis of pembrolizumab plus chemotherapy versus pembrolizumab monotherapy for advanced or recurrent non-small cell lung cancer. Thorac Cancer. 2021;12(9):1387–97. https://doi.org/10.1111/1759-7714.13915.
Ivanović M, Knez L, Herzog A, et al. Immunotherapy for metastatic non-small cell lung cancer: real-world data from an academic central and eastern European center. Oncologist. 2021;26(12):e2143–50. https://doi.org/10.1002/onco.13909.
Kawachi H, Tamiya M, Tamiya A, et al. Association between metastatic sites and first-line pembrolizumab treatment outcome for advanced non-small cell lung cancer with high PD-L1 expression: a retrospective multicenter cohort study. Invest New Drugs. 2020;38(1):211–8. https://doi.org/10.1007/s10637-019-00882-5.
Matsumoto H, Kobayashi N, Somekawa K, et al. Pembrolizumab monotherapy versus pembrolizumab plus chemotherapy in patients with non-small-cell lung cancer: a multicenter retrospective trial. Thorac Cancer. 2022;13(2):228–35. https://doi.org/10.1111/1759-7714.14252.
Metro G, Banna GL, Signorelli D, et al. Efficacy of pembrolizumab monotherapy in patients with or without brain metastases from advanced non-small cell lung cancer with a PD-L1 expression ≥50%. J Immunother. 2020;43(9):299–306. https://doi.org/10.1097/CJI.0000000000000340.
Metro G, Gili A, Signorelli D, et al. Upfront pembrolizumab as an effective treatment start in patients with PD-L1 ≥ 50% non-oncogene addicted non-small cell lung cancer and asymptomatic brain metastases: an exploratory analysis. Clin Transl Oncol. 2021;23(9):1818–26.
Mountzios G, de Toma A, Economopoulou P, et al. Steroid use independently predicts for poor outcomes in patients with advanced NSCLC and high PD-L1 expression receiving first-line pembrolizumab monotherapy. Clin Lung Cancer. 2021;22(2):e180–92. https://doi.org/10.1016/j.cllc.2020.09.017.
Mouritzen MT, Carus A, Ladekarl M, et al. Nationwide survival benefit after implementation of first-line immunotherapy for patients with advanced NSCLC-real world efficacy. Cancers (Basel). 2021;13(19):4846. https://doi.org/10.3390/cancers13194846.
Noordhof AL, Damhuis RAM, Hendriks LEL, et al. Prognostic impact of KRAS mutation status for patients with stage IV adenocarcinoma of the lung treated with first-line pembrolizumab monotherapy. Lung Cancer. 2021;155:163–9. https://doi.org/10.1016/j.lungcan.2021.04.001.
Passaro A, Novello S, Giannarelli D, et al. Early progression in non-small cell lung cancer (NSCLC) with high PD-L1 treated with pembrolizumab in first-line setting: a prognostic scoring system based on clinical features. Cancers (Basel). 2021;13(12):2935. https://doi.org/10.3390/cancers13122935.
Sánchez-Gastaldo A, Muñoz-Fuentes MA, Molina-Pinelo S, et al. Correlation of peripheral blood biomarkers with clinical outcomes in NSCLC patients with high PD-L1 expression treated with pembrolizumab. Transl Lung Cancer Res. 2021;10(6):2509–22.
Schakenraad A, Hashemi S, Twisk J, et al. The effect of tumor size and metastatic extent on the efficacy of first line pembrolizumab monotherapy in patients with high PD-L1 expressing advanced NSCLC tumors. Lung Cancer. 2021;162:36–41. https://doi.org/10.1016/j.lungcan.2021.10.002.
Takumida H, Horinouchi H, Masuda K, et al. Comparison of time to failure of pembrolizumab plus chemotherapy versus pembrolizumab monotherapy: a consecutive analysis of patients having NSCLC with high PD-L1 expression. Cancer Immunol Immunother. 2022;71(3):737–46. https://doi.org/10.1007/s00262-021-03029-9.
Tambo Y, Sone T, Shibata K, et al. Real-world efficacy of first-line pembrolizumab in patients with advanced or recurrent non-small-cell lung cancer and high PD-L1 tumor expression. Clin Lung Cancer. 2020;21(5):e366–79. https://doi.org/10.1016/j.cllc.2020.02.017.
Velcheti V, Chandwani S, Chen X, et al. Outcomes of first-line pembrolizumab monotherapy for PD-L1-positive (TPS ≥50%) metastatic NSCLC at US oncology practices. Immunotherapy. 2019;11(18):1541–54. https://doi.org/10.2217/imt-2019-0177.
Wakuda K, Yabe M, Kodama H, et al. Efficacy of pembrolizumab in patients with brain metastasis caused by previously untreated non-small cell lung cancer with high tumor PD-L1 expression. Lung Cancer. 2021;151:60–8. https://doi.org/10.1016/j.lungcan.2020.11.009.
Yamaguchi O, Kaira K, Shinomiya S, et al. Pre-existing interstitial lung disease does not affect prognosis in non-small cell lung cancer patients with PD-L1 expression ≥50% on first-line pembrolizumab. Thorac Cancer. 2021;12(3):304–13.
Juarez-Garcia A, Sharma R, Hunger M, et al. Real-world effectiveness of immunotherapies in pre-treated, advanced non-small cell lung cancer patients: a systematic literature review. Lung Cancer. 2022;166:205–20. https://doi.org/10.1016/j.lungcan.2022.03.008.
Petrillo LA, El-Jawahri A, Nipp RD, et al. Performance status and end-of-life care among adults with non-small cell lung cancer receiving immune checkpoint inhibitors. Cancer. 2020;126(10):2288–95. https://doi.org/10.1002/cncr.32782.
Middleton G, Brock K, Savage J, et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. Lancet Respir Med. 2020;8(9):895–904. https://doi.org/10.1016/S2213-2600(20)30033-3.
Huo G, Liu W, Chen P. Inhibitors of PD-1 in non-small cell lung cancer: a meta-analysis of clinical and molecular features. Front Immunol. 2022;13: 875093. https://doi.org/10.3389/fimmu.2022.875093.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Macioch T.: conceptualization, methodology, validation, formal analysis, writing—original draft, visualization, supervision; Krzakowski M.: conceptualization, validation, supervision; Gołębiewska K.: methodology, formal analysis, resources, data curation, writing—original draft, visualization; Dobek M.: methodology, formal analysis, resources, data curation, writing—original draft, visualization; Warchałowska N.: methodology, formal analysis, resources, data curation, writing—original draft, visualization; Niewada M.: conceptualization, methodology, validation, writing—original draft, supervision, project administration.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
HealthQuest is the health technology assessment consultancy supporting pharma manufacturers in reimbursement application and preparation of HTA dossiers. Professor Maciej Krzakowski declares conflict of interest in a form of honoraria for lectures and advisory boards from MSD, ROCHE, BMS, ASTRAZENECA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Macioch, T., Krzakowski, M., Gołębiewska, K. et al. Pembrolizumab monotherapy survival benefits in metastatic non-small-cell lung cancer: a systematic review of real-world data. Discov Onc 15, 303 (2024). https://doi.org/10.1007/s12672-024-01153-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01153-3