Abstract
Objective
To evaluate the diagnostic value of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) for intracapsular prostate cancer with a poor prognosis (PPC) and no extracapsular invasion or distant metastasis.
Methods
The PET/CT images and clinical data of 221 patients were retrospectively analyzed. These patients all had clear pathological results. The maximum standard uptake value (SUVmax) of the main lesions was measured at the postprocessing workstation and was tested for correlation with the pathological score. The diagnostic accuracy was calculated using the receiver operating characteristic (ROC) curve, and the best diagnostic threshold was calculated. The correlation between SUVmax and the International Society of Urological Pathology Grade Group (GG) was also analyzed.
Results
The pathological results of the 221 patients were 48 benign lesions and 173 malignant lesions, including 81 PPC. Low-, intermediate-, and high-risk prostate cancers made up 21.97% (38/173), 54.33% (94/173), and 23.70% (41/173) of the malignant lesions, respectively. SUVmax and GG were positively correlated (r = 0.54, P < 0.01). The best SUVmax thresholds for 68Ga-PSMA PET/CT for the diagnosis of intracapsular PC and PPC were 7.95 and 13.94, respectively; the specificities were 0.83 and 0.85, the negative predictive values were 0.55 and 0.87, and the areas under the ROC curves were 0.88 and 0.88, respectively.
Conclusion
68Ga-PSMA PET/CT has high specificity and NPV in the diagnosis of intracapsular PPC, but the sensitivity for the diagnosis of intracapsular low-risk PC is low, which may cause some cases to be undetected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Prostate cancer (PC) is the second most common malignant tumor in men worldwide (about 29%) [1]. With demographic aging and changes in people's lifestyles, PC morbidity and mortality are rising constantly. Therefore, accurate early detection and risk prediction are highly important for timely treatment and improvement of patient prognosis. This is especially true for intracapsular prostate cancer because when it spreads beyond the capsule or metastasizes, the treatment can become very ineffective. Studies are being performed on intermediate- to high-risk PC (IHPC), but IHPC also includes patients with grade group 2 (GG2). The risk level of these patients is usually lower than that of GG3 patients, and they are less likely to develop metastasis and recurrence. Therefore, we defined a poor prognosis prostate cancer (PPC) as that with GG ≥ 3, which has a significantly greater probability of metastasis and recurrence, a worse prognosis, and a lower 5-year survival rate than low-risk PC [2, 3]. PPC patients need more aggressive and longer-term radiation therapy and hormone therapy. Increasing the detection rate of intracapsular prostate cancer with poor prognosis before treatment helps patients receive the best treatment option in a timely manner and improves the survival rate. This topic still needs further study, so it is important to differentially diagnose them in clinical management.
Prostate-specific antigen (PSA) is often screened to diagnose PC, but its specificity is low, and it may be accompanied by overdiagnosis and overtreatment [4]. Transrectal ultrasound-guided biopsy (TRUS-GB) is the main method for the preoperative diagnosis of male prostate cancer with elevated serum PSA levels [5]. However, patients generally require hospitalization for examination, and missed diagnoses and complications can occur [6], such as bleeding, infection, etc. The pathological score of needle biopsy was inconsistent with the pathological score after radical resection. Imaging methods can help determine which males with elevated PSA continue to undergo biopsy, which may reduce unnecessary biopsies and improve diagnostic accuracy. Magnetic resonance imaging (MRI), a mature imaging method, is widely used in prostate examination [7] because the low signal density on T2-weighted images caused by stromal hyperplasia and chronic inflammation is similar to that on PC. Although its sensitivity is high, the specificity of MRI needs to be improved. Especially, small lesions and diseases within the central gland may not be easily identified by multiparameter MRI (mpMRI), and up to 35% of clinically significant prostate cancer may be invisible [8].
Prostate-specific membrane antigen (PSMA) is a type II transmembrane glycoprotein receptor found on the cell surface, and its expression is significantly elevated in more than 90% of prostate cancer cells (1000 times more than that in normal prostate cells). In recent years, targeted PSMA positron emission tomography/computed tomography (PET/CT) has shown relatively high value in the detection of PC metastasis and recurrent lesions [9,10,11,12]. As a new molecular analysis-based imaging method, it has higher specificity and sensitivity in detecting prostate tumor lesions and metastases compared to MRI. This application has been approved by the U.S. Food and Drug Administration, and its preliminary application has also begun in mainland China. PSMA PET/CT also has relatively high sensitivity and specificity in the diagnosis of primary PC, and its maximum standardized uptake value (SUVmax) is an independent predictor of clinically significant PC [13].
In patients with high-risk scores and without metastatic disease, radical prostatectomy is the preferred treatment [14]. PC patients with metastasis often have a poor prognosis. The identification of PC patients with a poor prognosis before metastasis helps to guide treatment and improve their prognosis [15]. These patients can benefit the most from imaging. Lymph node and bone metastasis are prone to occur in PC patients with a poor prognosis, and qualitative diagnosis of the primary lesion is relatively easy [16]. Intracapsular PC is an early prostate cancer, and the prognosis of radical resection is good; therefore, early diagnosis is highly important. It is difficult to predict and diagnose intracapsular PC patients with no metastasis.
Such an approach is more clinically significant and can guide clinicians in timely and active treatment, but there is no relevant literature investigating this issue. This study evaluated the diagnostic value of 68Ga-PSMA PET/CT for identifying intracapsular PC patients with a poor prognosis who did not have extracapsular invasion or distant metastasis.
2 Materials and methods
2.1 Clinical data
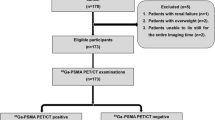
The data of patients who underwent 68Ga-PSMA PET/CT at our hospital between January 2017 and December 2023 were retrospectively analyzed. Each patient's medical history included difficulty urinating, elevated PSA, hematuria, and low-back pain. The inclusion criteria for the study were as follows: (1) suspected prostate cancer at the first visit (elevated PSA level, positive MRI, or positive digital examination), (2) an interval between 68Ga-PSMA PET/CT and radical resection of no more than 60 days, and (3) needle biopsy and radical resection. The exclusion criteria were as follows: (1) the patient had received androgen deprivation therapy, radiotherapy, chemotherapy, or prostate surgery before PET/CT examination; (2) had an interval between radical resection and 68Ga-PSMA PET/CT examination more than 60 days; (3) had prostate cancer invasion into extracapsular tissues found on radical mastectomy; and (4) had lymph node, bone, or lung metastatic lesions found during PET/CT examination or radical mastectomy. A total of 221 consecutive patients were included in the study. The patient flowchart is shown in Fig. 1. All patients signed informed consent before 68Ga-PSMA PET/CT examination, and the Ethics Committee of Nanjing First Hospital approved this retrospective study.
2.2 PET/CT examination and image analysis
68Ga-PSMA was synthesized in the class IV laboratory of our hospital using the automated labeling module of the International Thoracic Group (ITG), Germany. It was prepared following laboratory standards to radiochemical purity greater than 93%. 68Ga-PSMA was injected intravenously at an injection dose of approximately 2 MBq/kg. The patients were instructed to drink more water and urinate more often. The patient underwent spiral CT scan at approximately 45 min and PET scan (United Imaging, uMI780, Shanghai, China) at approximately 60 min. From the top of the cranium to the middle of the thigh, the PET data were collected from four beds, each bed taking approximately 3 min. The PET images and CT images were fused at the workstation to obtain multiplanar images, and the maximum standard uptake value was measured.
Before the pathological results were disclosed, two nuclear medicine physicians conducted blinded analyses of the PET images. The main diagnosis was based on the SUVmax of the main lesion. The main lesion was defined as that with the most obvious radioactive concentration. If no significant radioactive concentration was found in the prostate, then the area above background in the prostate with the highest SUVmax was selected as the target area of interest. The diameter of the measurement range was always greater than 1 cm. In this study, only lesions with a volume greater than 0.5 cm3 were included.
2.3 Pathology
All patients underwent transrectal ultrasound-guided needle biopsy (TUNB). The malignant patients underwent radical prostatectomy. During TUNB, six punctures were taken on the prostate on each side, and the serial number of each puncture was recorded (12 in total). The score for benign lesions (BLs) was based on the puncture result, and the score for malignant patients after radical resection was used as the cutoff. Benign prostatic hyperplasia (BPH) and prostatitis belonged to the BL subgroup, patients with BL and GG1-2 belonged to the nonhigh-risk subgroup, and patients with GG ≥ 3 belonged to the poor prognosis subgroup.
2.4 Statistical analysis
Normally distributed data are expressed as mean ± standard deviation, and the means were compared using Student’s t test. When the data did not fit a normal distribution, they are given as median (P25, P75), and the Mann‒Whitney U test compared their means. The relationships between the imaging and pathological results were assessed by Spearman correlation analysis. Statistical analysis was done with SPSS (version 25.0; IBM, Inc., Chicago, IL, USA) and GraphPad Prism (version 9.0; GraphPad Software, Inc., La Jolla, CA, USA). A receiver operating characteristic (ROC) curve was drawn and the area under the curve (AUC) calculated to determine the optimal SUVmax threshold. P < 0.05 indicated statistical significance.
3 Results
Pathology revealed 48 benign lesions and 173 malignant lesions in 221 patients, including 81 cases with poor prognosis. The distribution proportions and SUVmax of the lesions are given in Table 1. Low-, intermediate-, and high-risk PCs made up 21.97% (38/173), 54.33% (94/173), and 23.70% (41/173) of the malignant lesions, respectively. The PET images of benign lesions showed very low radiotracer concentrations, similar to or slightly greater than those in normal tissues, though a few benign lesions had a moderate or greater radioactive concentration. The radiotracer concentration was obvious on the PET images of the PCs (Fig. 2), and the median SUVmax was greater than that of the benign tissues (P < 0.05). A very few malignant lesions did not have a significant radioactive concentration. The interval between radical treatment and PET/CT examination was 8 ± 4 days. SUV and GG did not correlate with the mCi value of the tracer (P > 0.05). SUV and GG were each correlated with age (P < 0.05). Table 2 compares the median SUVmax between groups.
A 79-year-old male patient. Physical examination revealed elevated blood PSA (8.85 ng/ml). He did not usually have difficulty urinating. A nodular focus of higher radioactive uptake was observed in the left transition zone (a), with an SUVmax of 29.18 (b). Prostate cancer was confirmed by pathology: Gleason score: 3 + 4 = 7 (c)
The SUVmax and GG were positively correlated in the different risk groups (r = 0.54, P < 0.01) (Fig. 3). A box plot of the SUVmax in the different risk groups is shown in Fig. 4. Figure 5 shows the percentage of patients in each group with an SUVmax above and below the diagnostic threshold of PC, IHPC and PPC. According to the results of the ROC curve and 68Ga-PSMA PET/CT data, the best SUVmax thresholds for the diagnosis of intracapsular PC and PPC were 7.95 and 13.94, respectively. The detailed results are shown in Table 3. The ROC curves for the diagnosis of PC, IHPC and PPC are shown in Fig. 6. According to the ROC curve, when the SUVmax was 13.94, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), Youden index, and AUC of 68Ga-PSMA PET/CT for diagnosing PPC are 0.78, 0.85, 0.75, 0.87, 0.63, and 0.88, respectively.
4 Discussion
This study investigated the value of 68Ga-PSMA PET/CT for the preoperative diagnosis of intracapsular PPC. This technique can help improve the confidence of radiologists and clinicians in the diagnosis of PPC before radical prostatectomy and thus increase the reliability of delivery to patients. Diagnostic information can help clinicians and patients make the best choice when discussing treatment options [17]. This study showed that the specificity (85%) and NPV (87%) of 68Ga-PSMA PET/CT for the diagnosis of PPC in the capsule were relatively high, and the sensitivity (90%) and accuracy (AUC = 0.91) for the diagnosis of IHPC were high. For intracapsular PPC, high specificity and NPV usually mean high clinical value, which can be reflected in the following aspects. When PET/CT imaging is negative for prostate cancer lesions, it is highly reliable in ruling out the presence of patients with metastasis. This can provide more accurate disease staging information, which is crucial for determining the accurate location and extent of tumors. This provides patients with more treatment options and to some extent reduces the occurrence of unnecessary biopsies or chemotherapy, reducing the potential treatment-related complications and adverse effects that patients may face, helping improve their quality of life.
Although the 10-year survival rate of PC is approximately 90%, advanced PC may be life-threatening, especially in the metastatic stage. Mottet N et al. [5] showed that PSA screening alone detected more low-risk PC patients but had no significant effect on PC mortality (median follow-up of 10 years), indicating that these patients may have received unnecessary treatment. TRUS needle biopsy may detect no clinically significant PCs and miss the diagnosis of clinically significant prostate cancers (up to 30%), resulting in imprecise risk stratification. However, compared with TRUS-guided biopsy, mpMRI-targeted biopsy yields 20% more clinically significant prostate cancers [18]. Due to the low specificity of mpMRI, the mpMRI results of low-risk patients might lead to unnecessary biopsies caused by false positives. Currently, mpMRI is not recommended as an initial screening tool before needle biopsy [19].
The diagnostic threshold and the median in the present study were slightly lower than those in our previous study. Previous studies included all PCs, so some of the patients had distant metastasis and extracapsular invasion. These patients had a greater degree of malignancy than the other patients and had no early lesions at the time of diagnosis, so qualitative diagnosis was easier. The main role of the examination was to perform a staging evaluation, but the diagnostic threshold was higher. One of the most important clinical tasks when encountering lesions without extracapsular invasion or distant metastasis is to perform qualitative assessment and predictive risk stratification, which was also the main purpose of this study. We studied intracapsular PC, and easy-to-diagnose intermediate to advanced PC patients were not included. Therefore, the proportion of our patients with low- and intermediate-risk PC with relatively slow progression was greater (approximately 81%). The results of this study differ from those of previous studies [20], whose threshold and median values are higher than us(threshold:8 vs 7.95; median:26.1 vs 13.68). The result of this study can provide guidance for when early intracapsular PC is encountered in clinical practice.
The SUVmax is a quantifiable objective indicator and one of the main reference standards for subjective diagnosis. The range of SUVmax is 0 to 100. It is difficult to visually detect gray-level differences in images with SUVmax above 10; such differences can be detected only through computerized measurements, which are different from those of MR T2W and apparent diffusion coefficient (ADC) images. The gray-level variation range and the difference in the extreme values of the ADC values of the MR images are small, so the differences can be identified visually. Therefore, the magnetic resonance Prostate Imaging-Reporting and Data System (PI-RADS) diagnostic criteria have been recognized worldwide, but the empirical and subjective diagnostic criteria for PSMA-RADS (Prostate-Specific Membrane Antigen Reporting and Data System) reliability still need extensive validation [19, 21]. PSMA-RADS is a standardized system for interpreting 68Ga-PSMA PET/CT imaging in patients with prostate cancer. It provides a structured approach to reporting imaging findings, which can help in better interpretation and communication among healthcare providers, but there are some potential shortcomings to consider. Most importantly, it may not be widely adopted or standardized across all institutions. Variability in reporting practices may exist between different institutions, making it challenging to compare results across centers. Though PSMA-RADS provides a structured approach to interpreting 68Ga-PSMA PET/CT imaging, its clinical utility may be influenced by potential limitations. Continued research and validation are necessary to further refine and optimize its use in clinical practice [22, 23].This study found that SUVmax was positively correlated with PC risk. However, the median SUVmax of high-risk PCs was the highest at GG3, whereas the SUVmax at GG4 and 5 gradually decreased, which was not the expected increase in SUVmax with increasing score. This difference may be related to the greater degree of malignancy of IHPC and the reduced expression level of PSMA.
The aim of the current study was assessment of involved intracapsular lesions using 68Ga-PSMA PET/CT imaging. Most of these malignant lesions were still in the early stage of development, and the peak time of their malignant characteristics had not yet been reached. Previous studies [24,25,26] have fully discussed the diagnostic value of PET/CT for PC patients with metastasis, which is more easily diagnosed in clinical practice. When patients occur metastasis, their prognosis are often poor with limited treatment options and decreased survival rates. Our article mainly discusses the diagnostic value of PET/CT for patients without metastasis, whose malignancy is not as high as those with metastasis, and because there is no metastasis or extracapsular invasion, it is often impossible to have clear qualitative staging results even after receiving MRI examination. These patients require puncture biopsy to determine staging, but this is an invasive examination that can cause certain risks and adverse reactions to patients. PSMA is a highly specific targeted probe, particularly valuable in staging and grading. So this article mainly explores the performance of PET/CT in diagnosing intracapsular PPC, and finds that it has high specificity and NPV, therefore they can be detected early and timely, which will be more beneficial for the prognosis of patients and the selection of clinical plans. This study showed that the reliability of 68Ga-PSMA PET/CT for detecting low-risk PC was low; and even though BL and low-risk PC could not be clearly distinguished, the diagnostic reliability of high-risk PC was high [27]. Our study found that PET/CT has a relatively low NPV in diagnosing low-risk PC, resulting in poor diagnostic reliability. This is also the main limitation of 68Ga-PSMA PET/CT examination method [28, 29]. Firstly, it depends on the expression level of PSMA, but not all prostate cancer cells are highly expressing PSMA, especially low-grade tumors may have lower PSMA expression. And the spatial resolution of PET/CT imaging is limited, which may result in poor detection performance for small lesions with lower radioactive condensation. Therefore, for small or low metabolic activity prostate cancer lesions, PET/CT imaging may not be able to detect them, leading to an increased false negative rate of negative results. In addition, false positives caused by prostatitis or infection may also be another reason for low NPV although its occurrence rate is relatively low. Inflammatory reactions can increase the metabolic activity of local prostate tissue, leading to an increase of PSMA expression in prostate tissue. This may be mistaken for prostate cancer lesions, resulting in false positive results in PET/CT imaging.
This study once again confirms that SUVmax has a high positive predictive value and AUC for the diagnosis of all PCs and PPCs and demonstrated its value for the differentiation of patients with GG2 and GG3 [30]. The SUVmax of the GG3 PCs was significantly greater than that of the GG2 PCs and was close to the median of the PPCs. If these GG3 patients are included in the high-risk prostate cancer group, the diagnostic performance of the SUVmax could be significantly improved, which again indirectly confirmed the significance of GG3. These patients have a worse than GG2 patients, so we considered GG3 patients to PPC with worse prognosis [31, 32].
Our study has some limitations. The first limitation is that we studied a single-center model, and the reference threshold for the SUVmax obtained was not universal. This is also a shortcoming shared by other studies [13, 33]. Because the models used in each research center differ, the diagnostic thresholds for benign and malignant prostate lesions in the published literature are between 4 and 11, and each threshold can be used only as a diagnostic reference for one research center, which is also one of the reasons to establish a PSMA-RADS diagnostic standard. To overcome this limitation, a multicenter study needs to analyze the causes of the differences in the threshold under the conditions of uniform drug concentration and injection volume. Additionally, one of the drawbacks of PET is that its resolution is not as high as MRI's, we do not recommend using PSMA PET as the preferred examination method for primary PC. We prioritize economical and radiation free MRI techniques. But PSMA PET has targeted imaging characteristics that MRI does not have, and can compensate for the shortcomings of MRI. Therefore, in order to detect PPC as soon as possible, we recommend performing PSMA PET examination when PC is suspected but MRI examination is ambiguous.
5 Conclusion
68Ga-PSMA PET/CT has high specificity and NPV in diagnosing intracapsular PPC, but its sensitivity for diagnosing intracapsular low-risk PC is low, which may cause some early low-risk PCs to be missed.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. https://doi.org/10.3322/caac.21763.
Martin RM, Donovan JL, Turner EL, et al. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883–95. https://doi.org/10.1001/jama.2018.0154.
Gillessen S, Bossi A, Davis ID, et al. Management of patients with advanced prostate cancer. Part i: intermediate-/high-risk and locally advanced disease, biochemical relapse, and side effects of hormonal treatment: report of the advanced prostate cancer consensus conference 2022. Eur Urol. 2023;83:267–93. https://doi.org/10.1016/j.eururo.2022.11.002.
Van Poppel H, Albreht T, Basu P, et al. Serum PSA-based early detection of prostate cancer in Europe and globally: past, present and future. Nat Rev Urol. 2022;19:562–72. https://doi.org/10.1038/s41585-022-00638-6.
Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62. https://doi.org/10.1016/j.eururo.2020.09.042.
Buyyounouski MK, Choyke PL, McKenney JK, et al. Prostate cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:245–253. https://doi.org/10.3322/caac.21391
Stabile A, Giganti F, Rosenkrantz AB, et al. Multiparametric MRI for prostate cancer diagnosis: current status and future directions. Nat Rev Urol. 2020;17:41–61. https://doi.org/10.1038/s41585-019-0212-4.
Panebianco V, Barchetti G, Simone G, et al. Negative multiparametric magnetic resonance imaging for prostate cancer: What’s next? Eur Urol. 2018;74:48–54. https://doi.org/10.1016/j.eururo.2018.03.007.
Pepe P, Pennisi M. Should 68Ga-PSMA PET/CT replace CT and bone scan in clinical staging of high-risk prostate cancer? Anticancer Res. 2022;42:1495–8. https://doi.org/10.21873/anticanres.15621.
Huo H, Shen S, He D, et al. Head-to-head comparison of 68Ga-PSMA-11 PET/CT and 68Ga-PSMA-11 PET/MRI in the detection of biochemical recurrence of prostate cancer: summary of head-to-head comparison studies. Prostate Cancer Prostatic Dis. 2023;26:16–24. https://doi.org/10.1038/s41391-022-00581-y.
Chow KM, So WZ, Lee HJ, et al. Head-to-head comparison of the diagnostic accuracy of prostate-specific membrane antigen positron emission tomography and conventional imaging modalities for initial staging of intermediate- to high-risk prostate cancer: a systematic review and meta-analysis. Eur Urol. 2023;84:36–48. https://doi.org/10.1016/j.eururo.2023.03.001.
Franklin A, Yaxley WJ, Raveenthiran S, et al. Histological comparison between predictive value of preoperative 3-T multiparametric MRI and 68 Ga-PSMA PET/CT scan for pathological outcomes at radical prostatectomy and pelvic lymph node dissection for prostate cancer. BJU Int. 2021;127:71–9. https://doi.org/10.1111/bju.15134.
Jiao J, Kang F, Zhang J, et al. Establishment and prospective validation of an SUVmax cutoff value to discriminate clinically significant prostate cancer from benign prostate diseases in patients with suspected prostate cancer by 68Ga-PSMA PET/CT: a real-world study. Theranostics. 2021;11:8396–411. https://doi.org/10.7150/thno.58140.
Preisser F, Bandini M, Marchioni M, et al. Extent of lymph node dissection improves survival in prostate cancer patients treated with radical prostatectomy without lymph node invasion. Prostate. 2018;78:469–75. https://doi.org/10.1002/pros.23491.
Murthy V, Sonni I, Jariwala N, et al. The role of PSMA PET/CT and PET/MRI in the initial staging of prostate cancer. Eur Urol Focus. 2021;7:258–66. https://doi.org/10.1016/j.euf.2021.01.016.
Lenis AT, Pooli A, Lec PM, et al. Prostate-specific membrane antigen positron emission tomography/computed tomography compared with conventional imaging for initial staging of treatment-naïve intermediate- and high-risk prostate cancer: a retrospective single-center study. Eur Urol Oncol. 2022;5:544–52. https://doi.org/10.1016/j.euo.2020.08.012.
Donato P, Roberts MJ, Morton A, et al. Improved specificity with 68Ga PSMA PET/CT to detect clinically significant lesions “invisible” on multiparametric MRI of the prostate: a single institution comparative analysis with radical prostatectomy histology. Eur J Nucl Med Mol Imaging. 2019;46:20–30. https://doi.org/10.1007/s00259-018-4160-7.
Roethke M, Anastasiadis AG, Lichy M, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol. 2012;30:213–8. https://doi.org/10.1007/s00345-011-0675-2.
Emmett L, Buteau J, Papa N, et al. The additive diagnostic value of prostate-specific membrane antigen positron emission tomography computed tomography to multiparametric magnetic resonance imaging triage in the diagnosis of prostate cancer (PRIMARY): a prospective multicentre study. Eur Urol. 2021;80:682–9. https://doi.org/10.1016/j.eururo.2021.08.002.
Pepe P, Pepe L, Tamburo M, et al. 68Ga-PSMA PET/CT and prostate cancer diagnosis: Which SUVmax value? In Vivo. 2023;37:1318–22. https://doi.org/10.21873/invivo.13211.
Chavoshi M, Mirshahvalad SA, Metser U, Veit-Haibach P. 68Ga-PSMA PET in prostate cancer: a systematic review and meta-analysis of the observer agreement. Eur J Nucl Med Mol Imaging. 2022;49:1021–9. https://doi.org/10.1007/s00259-021-05616-5.
Werner RA, Hartrampf PE, Fendler WP, et al. Prostate-specific membrane antigen reporting and data system version 2.0. Eur Urol. 2023;84:491–502. https://doi.org/10.1016/j.eururo.2023.06.008.
Rauscher I, Maurer T, Fendler WP, et al. (68)Ga-PSMA ligand PET/CT in patients with prostate cancer: How we review and report. Cancer Imaging. 2016;16:14. https://doi.org/10.1186/s40644-016-0072-6.
Meijer D, Ettema RH, van Leeuwen PJ, et al. The prognostic value of lymph node staging with prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) and extended pelvic lymph node dissection in node-positive patients with prostate cancer. BJU Int. 2023;131:330–8. https://doi.org/10.1111/bju.15881.
Mj H, Wi L, A J, et al. The diagnostic value of PSMA PET/CT in men with newly diagnosed unfavorable intermediate-risk prostate cancer. J Nuclear Med. 2023. https://doi.org/10.2967/jnumed.122.265205.
Nuo Y, Li A, Yang L, et al. Efficacy of 68Ga-PSMA-11 PET/CT with biparametric MRI in diagnosing prostate cancer and predicting risk stratification: a comparative study. Quant Imaging Med Surg. 2022;12:53–65. https://doi.org/10.21037/qims-21-80.
Scheltema MJ, Chang JI, Stricker PD, et al. Diagnostic accuracy of 68 Ga-prostate-specific membrane antigen (PSMA) positron-emission tomography (PET) and multiparametric (mp)MRI to detect intermediate-grade intra-prostatic prostate cancer using whole-mount pathology: impact of the addition of 68 Ga-PSMA PET to mpMRI. BJU Int. 2019;124(Suppl 1):42–9. https://doi.org/10.1111/bju.14794.
Barrio M, Fendler WP, Czernin J, Herrmann K. Prostate specific membrane antigen (PSMA) ligands for diagnosis and therapy of prostate cancer. Expert Rev Mol Diagn. 2016;16:1177–88. https://doi.org/10.1080/14737159.2016.1243057.
Giesel FL, Sterzing F, Schlemmer HP, et al. Intra-individual comparison of (68)Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:1400–6. https://doi.org/10.1007/s00259-016-3346-0.
Yi N, Wang Y, Zang S, et al. Ability of 68 Ga-PSMA PET/CT SUVmax to differentiate ISUP GG2 from GG3 in intermediate-risk prostate cancer: a single-center retrospective study of 147 patients. Cancer Med. 2023;12:7140–8. https://doi.org/10.1002/cam4.5516.
Hoffmann MA, Miederer M, Wieler HJ, et al. Diagnostic performance of 68Gallium-PSMA-11 PET/CT to detect significant prostate cancer and comparison with 18FEC PET/CT. Oncotarget. 2017;8:111073–83. https://doi.org/10.18632/oncotarget.22441.
Hashmi AA, Iftikhar SN, Munawar S, et al. International society of urological pathology (ISUP)-grade grouping in prostatic adenocarcinoma and its prognostic implications. Cancer Invest. 2022;40:211–8. https://doi.org/10.1080/07357907.2021.2019263.
Mj H, De O-L, An V, et al. Reproducibility of PSMA PET/CT imaging for primary staging of treatment-naïve prostate cancer patients depends on the applied radiotracer: a retrospective study. J Nucl Med. 2022. https://doi.org/10.2967/jnumed.121.263139.
Acknowledgements
We sincerely thank all support from all authors.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by YJW, JPS, LLY, and WCL. The first draft of the manuscript was written by YJW, JPS, and LLY. LWW, WW, AQJ and FW revised the work critically for important intellectual content. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This retrospective study was also approved by the Ethics Committee of the Nanjing First Hospital (KY20171208-03) and the requirement for informed consent was waived. The ethical approval was in Related files. All methods were carried out in accordance with relevant guidelines and regulations.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Song, J., Yang, L. et al. The value of 68Ga-PSMA PET/CT in the diagnosis of intracapsular prostate cancer with a poor prognosis. Discov Onc 15, 252 (2024). https://doi.org/10.1007/s12672-024-01127-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-024-01127-5