Abstract
RNA modification is a post-transcriptional level of regulation that is widely distributed in all types of RNAs, including mRNA, tRNA, rRNA, miRNA, and lncRNA, where N6-methyladenine (m6A) is the most abundant mRNA methylation modification. Significant evidence has depicted that m6A modifications are closely related to human diseases, especially cancer, and play pivotal roles in RNA transcription, splicing, stabilization, and translation processes. The most common urological cancers include prostate, bladder, kidney, and testicular cancers, accounting for a certain proportion of human cancers, with an ever-increasing incidence and mortality. The recurrence, systemic metastasis, poor prognosis, and drug resistance of urologic tumors have prompted the identification of new therapeutic targets and mechanisms. Research on m6A modifications may provide new solutions to the current puzzles. In this review, we provide a comprehensive overview of the key roles played by RNA modifications, especially m6A modifications, in urologic cancers, as well as recent research advances in diagnostics and molecularly targeted therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
All urological cancers account for 13% of incidence and 18% of mortality globally. Prostate cancer (PCa) is one of the most common cancers in men and in urology, accounting for 56% of all urological cancers, followed by bladder cancer (BCa), ranked the second most common and fatal cancer, with approximately four times higher mortality in men compared to in women. Renal cell carcinoma (RCC) has an incidence and mortality rate of 17% and 23%, respectively [1,2,3], while testicular cancer (TC) is the most common malignancy in men aged 15–35 years, with germ cell tumors (GCT) accounting for the majority of TC [4]. Surgery has been regarded as the most effective treatment strategy for urological tumors, but the five-year survival rates remain unsatisfactory [5, 6]. Similarly, androgen deprivation therapy (ADT), combined with a novel endocrine adjuvant therapy, is often employed for locally progressive and metastatic prostate cancer [7,8,9]. Targeted therapies at the molecular level have changed the management paradigm for patients with RCC, with different regimens developed and approved for treating patients with advanced RCC. However, their realization remains limited because of various factors [10,11,12,13]. BCa patients have a higher recurrence rate, are prone to distant metastases, and have a poorer prognosis [14]. Similarly, cisplatin resistance-elicited deaths in TC patients due to metastatic disease are still the main challenge [15]. Although many studies have been conducted to advance our understanding of urological cancers, they are still insufficient to explain the specific mechanisms clearly and provide good treatment strategies.
Transcriptional, post-transcriptional, and post-translational modifications are three levels of epigenetic modifications and changes, including DNA methylation, histone modifications, and chromatin remodeling [16, 17]. Post-transcriptional regulation includes RNA modifications and non-coding RNAs [18], and more than 170 RNA modifications have been identified to date [19], among which m6A,5-methylcytosine (m5C), N1-methyladenosine (m1A), 2'-O methylation (m6Am), pseudouridine (Ψ) and N7-Methylguanosine (m7G) are the most studied RNA epigenetic modifications [20,21,22,23,24,25] (see Fig. 1). They have been reported to regulate several critical cellular functions through their regulatory factors during development and disease. Although RNA modifications were discovered as early as the 1990s, RNA modifications still face several understanding gaps [26,27,28,29]. With the discovery of RNA demethylases and the application of methylation RNA immunoprecipitation sequencing technology, the role of RNA modifications in physiology and pathology has become a research hotspot [30].
m6A is one of the most common internal modifications in messenger RNA (mRNA) and is available in many species [31, 32]. It regulates the self-renewal of embryonic stem cells and cancer cells and facilitates survival after heat shock and DNA damage [31, 33, 34]. The occurrence of m6A modifications in the transcriptome was not random. The m6A modification sites have the typical recognition sequence DRACH (D = G, A, or U; R = G or A; H = A, C, or U) and are enriched in the coding sequence region (CDS) and 3'UTR [35]. m6A is present in almost all types of RNAs, including mRNAs, ribosomal RNAs (rRNAs), long-stranded non-coding RNAs (lncRNAs), miRNAs, small nuclear RNAs (snRNAs), and circular RNAs (circRNAs), and it is dynamically regulated in many physiological and pathological processes, including cancer [36,37,38,39,40,41,42,43].
In this review, we will discuss the role of RNA modifications in urologic cancers. We summarize recent studies that elucidate the therapeutic potential of targeting their aberrant deposition in cancers. m6A modification, one of the most widely studied modifications, embodies an important role in the development and progression of urologic cancers. Therefore, we will focus on the key role played by m6A in urologic cancers.
2 RNA methylation modifications
RNA methylation is a chemical modification in which methyl adenine of RNA is selectively added to methyl groups, catalyzed by methyltransferases [44]. In biology, methylation causes epigenetic changes and regulates their expression but does not affect gene sequences, which can occur in DNA, RNA, and proteins [45]. In this section, we introduce common types of RNA methylation other than m6A.
2.1 m5C modification
The m5C modification was first discovered in the 1970s by adding a living methyl group (mainly S-adenosylmethionine) from a donor to the carbon 5 position of cytosine in RNA [46]. Studies initially focused on tRNA and rRNA for m5C modifications [47], wherein the former is involved in optimizing codon and anticodon pairing, maintaining homeostasis, regulating stress responses, and controlling translation efficiency and accuracy [48,49,50,51,52,53], and plays a vital role in enhancing bacterial drug resistance [54, 55]. Similar to m6A methylation, regulatory RNA m5C levels can be classified by function into "writers,” "erasers," and "readers " proteins.
The m5C methylation "writers" protein of human RNA mainly comprises the NSUN family and DNMT2 [56]. The NSUN family catalyzes methyl transfer via a shared mechanism of covalent binding between the cysteine of the methyltransferase and cytosine in RNA to form a covalent intermediate, followed by nucleophilic addition of the electron-rich cytosine ring to the methyl group on S-adenosylmethionine (SAM) to complete methylation [57]. The most extensively studied RNA m5C methyltransferases in recent years are the NSUN family proteins, comprising nine members. Several have catalytic and release sites for methylation transferases, the most prominent and well-defined of which is NSUN2 [21]. SSUN2 is encoded by the NSUN2 gene on chromosome 10 and is a nucleolar RNA methyltransferase that catalyzes the m5C methylation of tRNA, mRNA, and ncRNA [58]. The SSUN2 mainly depends on two cysteine sites to function as a methyltransferase, with the C321 site catalyzing the methylation of cytosine by binding to the pyrimidine ring of cytosine to form a covalent bond and the C271 site mediating the release of RNA after methylation. The intracellular localization of NSUN2 in human epidermal cells was found to vary in different cell cycles [59, 60]. It is mainly distributed in the nucleus in G1 phase, between the nucleolus and nucleoplasm in S phase, localized in the cytoplasm in G2 phase, and in the centriole in M phase [61].
Similarly, NSUN1 and NSUN3-7 have also been demonstrated to bind RNAs, each catalyzing the methylation of different RNAs. NSUN1 is a nucleoprotein that primarily catalyzes the methylation of yeast 25S rRNA, 60S ribosomal subunit, and 26S rRNA[62]. NSUN3 is localized in the mitochondrial matrix of human and mouse cells and recognizes the anticodon loop of mitochondrial methionine transfer RNA (tRNAMet), methylating the C34 site [63]. NSUN4 is an rRNA-specific RNA methyltransferase that mainly acts on 12S rRNA in eukaryotic mitochondria [64, 65], while NSUN5 is a methyltransferase responsible for modifying the second m5C position in eukaryotic rRNAs and maintaining its high-level structure by catalyzing 28S and 25S rRNA methylation in nematodes, Drosophila, yeast, and plants [66]. NSUN6 is present in the eukaryotic cytoplasm and is partially localized in the Golgi apparatus, catalyzing the methylation of the 3'-UTRs C72 site of tRNA(Cys) (cysteinyl transfer RNA) and tRNA(Thr) (threonine transfer RNA) [67]. In contrast, NSUN7 silencing in human hepatocytes significantly reduced cytosine methylation levels of PFKL, SIRT5, IDH3B, and HMOX, suggesting that NSUN7 may act on eukaryotic eRNAs [68]. DNMT2 is also an m5C methyltransferase that improves tRNA stability and influences the expression and precision of protein synthesis, thereby ensuring precise peptide synthesis by recognizing near-homologous codons [50, 53, 69].
The process of DNA demethylation and TET family of demethylases are well known. The primary recognized mechanism is that TET catalyzes the m5C demethylation on DNA under the synergistic action of α-ketoglutarate and divalent iron ions [70,71,72]. It was recently found that knocking down TET2 in mouse embryonic stem cells resulted in a significant decrease in hm5C levels on tRNA, while its overexpression resulted in increased hm5C levels and decreased m5C levels on tRNA, and in vitro studies have revealed that oxidation of m5C modifications on tRNA catalyzed by TET2 also promoted the translation process [73]. The hydroxylation of m5C modification at the C34 site of mitochondrial tRNA to hm5C was also found to be mediated by another demethylase, ALKBH1, where its knockdown can result in impaired mitochondrial translation and respiratory function [74].
The reported m5C methylation recognition proteins mainly include ALYREF, RAD52, and YBX1, where ALYREF regulates nucleation, RAD52 is involved in DNA damage repair, and RNA stability is regulated by YBX1 [75]. ALYREF, a recognition protein for m5C methylation, can specifically bind to mRNAs with m5C modifications in the nucleus to form a complex of mRNPs to promote mRNA outgrowth. In ALYREF knockdown cell lines, mRNAs with mC modification accumulated in the nucleus, which ALYREF could only abolish in the back-complemented wild type. In contrast, ALYREF in the back-complemented mutant type remained unchanged, further verifying that ALYREF promotes mRNA outgrowth by recognizing and binding to mRNAs with mC modifications [75]. YBX1 is a well-known multifunctional DNA- and RNA-binding protein found in early zebrafish embryos that recognizes and binds m5C-modified mRNA through the Trp45 residue of the YBX1 cold excitation domain [76, 77].
2.2 m1A modification
N1-methyladenine (m1A) is an essential post-transcriptional RNA modification formed by adding methyl to the N1 position of adenosine [22]. The m1A methylation modification was discovered in the 1960s [78], which was previously thought to occur mainly in rRNA and tRNA, in maintaining RNA tertiary structure and affecting protein translation efficiency [79,80,81]. Recently, high-throughput sequencing has revealed that m1A modifications are also present in mRNA and can interfere with the Watson–Crick base-pairing principle, suggesting an important regulatory role for m1A modifications [82, 83].
The m1A-modified "writer" proteins included TRMT6, TRMT61A, TRMT61B, TRMT10C, and NML. TRMT61A, a catalytic subunit, contains a SAM-binding domain forming a functional complex with TRMT6 that binds to tRNA [84, 85], whereas TRMT10C and TRMT61B catalyze m1A modifications in mitochondrial tRNA [86, 87]. Recent studies have shown that these tRNA methyltransferases may also catalyze m1A mRNA [83, 88]. NML is located in the nucleus and methylesterase on m1A of 28S rRNA [89]. ALKBH1, ALKBH3, and FTO can demethylate m1A [74, 90, 91]. IYT521-B homolog (YTH) family proteins were recently identified as "readers" of m1A modifications. However, they also play a major role in m6A modifications [88, 92]. These include YTHDF1/2/3 and YTHDC1, which bind to the m1A site with weaker affinity than m6A; hence, their function as m1A readers still require further investigation [22].
2.3 2'-O methylation (m6Am)
The m6Am methylation modification is a chemical modification in which rRNA is methylated at the 2' position by RNA methylesterase [23]. m6Am methylation modifications are widely distributed in mRNA, tRNA, rRNA, miRNA, and other molecules [93]. It has been disclosed that m6Am methylation affects the binding of mRNA to proteins, regulates the translation efficiency of rRNA, and participates in biological processes, such as tRNA recognition [94]. Recent studies have identified phosphorylated CTD interaction factor 1 (PCIF1) as a methyltransferase of m6Am [95,96,97], in addition to FTO, which also acts as a demethylase of m6Am [91, 98].
2.4 m7G modification
m7G is one of the most abundant modifications in tRNA, rRNA, and mRNA, playing important roles in regulating RNA processing, metabolism, and function [25] and also occurs in miRNAs [99, 100]. In mammals, the METTL1 regulates m7G, which binds to the corresponding WD repeat domain 4 (WDR4), mediating m7G modifications in tRNA, miRNA, and mRNA [101]. RNA guanine-7 methyltransferase (RNMT) and its cofactor RNA guanine-7 methyltransferase (RAM) are involved in m7G modification at the 5' cap end of mRNA [102]. It has been reported that Williams-Beuren syndrome chromosome region 22 (WBSCR22) and tRNA methyltransferase activator subunit 11–2 (TRMT112) mediate m7G methylation in rRNA [103].
2.5 Ψ, Pseudouridine
Pseudouridine is the "fifth nucleotide" of RNA and is the most abundant RNA modification generally produced by uridine isomerization [104,105,106]. The pseudouridine of mRNA performs codon alteration, splicing, transcript stability enhancement, peptide bond formation, and stress responses [104, 107,108,109,110]. The pseudouridylation of RNA in eukaryotes mainly follows two pathways, that is, an RNA-dependent mechanism catalyzed mainly by DKC1, which forms a complex with box H/ACA snRNA, pseudouridylates RNA, and mediates post-transcriptional modifications of RNA, and an RNA non-dependent mechanism, which is directly recognized and catalyzed by an independent pseudouridine synthase substrate [111,112,113,114]. Specific "eraser" and "reader" for Ψ have still not been discovered [115, 116].
3 m6A modification
The m6A, a well-known post-transcriptional modification, was first discovered in 1974 and is the most abundant internal modification in mRNA, with approximately 25% of mRNAs carrying at least one m6A site [26, 117, 118]. m6A modifications are usually enriched at 3'UTRs, near-stop codons, long inner exons, intergenic regions, introns, and 5′ UTRs [119, 120]. Most approaches to m6A detection rely on the immunoprecipitation of methylated RNA using m6A recognition-specific antibodies, followed by a UV cross-linking step to bind the methylated RNA to the antibody, thereby allowing recognition of the m6A site [35, 119, 121, 122]. The mechanism of m6A methylation modification regulation is a dynamic process, catalyzed by m6A methyl transfer "writers,” removed by "erasers,” and finally recognized and bound by m6A "readers" to direct the translation and degradation of downstream mRNAs (See Fig. 2 and Table 1).
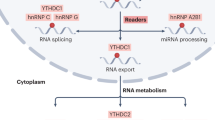
m6A RNA methylation and m6A modification mechanism. METTL3, METTL14, and WTAP form the core component of the methyltransferase complex and catalyze the methylation of N6 adenosine with other regulatory cofactors VIRMA, RBM15, ZC3H13, and METTL16. However, the deposition of m6A is reversible and dependent on the demethylases FTO and ALKBH5. m6A binding proteins can also recognize m6A. YTHDC1 can alternative splicing and RNA export; YTHDF1/2/3, eIF3 regulates RNA translation and degradation. IGFBP1/2/3 promotes RNA stability. hnRNPG/C and hnRNPA2B1 can regulate mRNA splicing.
3.1 m6A “writers”
m6A methylation is catalyzed by the methyltransferase complex (MTC), which catalyzes the methyl transfer of S-adenosylmethionine (SAM) to the nitrogen atom at position 6 of adenine [123]. The core component of MTC is methyltransferase-like protein 3 (METTL3), and the remaining components include METTL14, METTL16, WTAP, VIRMA, RBM15, and ZC3H13 [124,125,126,127]. METTL3 possesses a methyltransferase structural domain (MTD) that binds and catalyzes the transfer of methyl in SAM to the adenine base of RNA for the protein production of methionine homocysteine (SAH) [128]. Both METTL14 and METTL3 synergistically induce m6A modification [129]. The methyltransferase structural domain of METTL16 contains the Rossmann-like fold of class I methyltransferases with SAM as the methyl donor [130], whereas WTAP serves as an important cofactor for MTC [131]. VIRMA is involved in the recruitment and direction of core methyltransferase components to specific regions of mRNA [132], and ZC3H13 assists WTAP-RBM15 complex localization to the nucleus [133].
3.2 m6A “erasers”
m6A demethylation removes m6A methylation modifications and co-regulates m6A modifications with m6A methyltransferases to achieve a dynamic balance in m6A methylation modification levels in vivo [124, 134]. The two identified demethylases include FTO and ALKBH5, two mutually independent demethylases localized in the nucleus and belonging to the α-ketoglutarate dioxygenase family, catalyzing m6A demethylation in a Fe(II)- and α-ketoglutarate-dependent manner, respectively. Mechanistically, m6A is oxidized to N6-hydroxymethyl nonanoic acid (hm6A), which is then converted to N6-formyl adenosine (f6A), which is finally converted to adenosine [135]. FTO can catalyze the demethylation of m6A modification in addition to m6Am, reducing m6A and m6Am methylation levels on mRNA [136].
3.3 m6A “readers”
The m6A methyl recognition protein selectively recognizes and binds to m6A methylation modifications in target RNAs and participates in various stages of downstream target RNA metabolism [137,138,139]. The YTH family proteins include m6A YTH binding protein 1/2/3 (YTHDF1/2/3) and YTH structural domain protein 1/2 (YTHDC1/2) [140], where YTHDF1 and YTHDF3 interact through the translation of eIF3 and eIF4A3, resulting in increased translation efficiency of mRNAs [141]. YTHDF2 relocates, translating mRNAs from the cytoplasm to intracellular mRNA degradation sites and promoting mRNA degradation [142]. Similarly, YTHDC1 binds to sites on hnRNA where m6A methylation modifications occur, facilitating hnRNA splicing and processing of mRNA and facilitating mRNA export from the nucleus [143]. YTHDC2 selectively binds to m6A modification sites in the typical sequence of m6A and enhances the translation efficiency of target mRNAs [144]. hnRNP superfamily proteins include hnRNPA2B1 and hnRNPC/G [145], where the former recognizes m6A modifications in primary miRNA transcripts and interacts with DGCR8 to facilitate the processing of miRNAs [36]; the latter does not directly bind to m6A modification sites, but recognizes methylation modifications and then regulates mRNA abundance and splicing [41, 145]. By recognizing m6A modifications under normal and stress conditions, IGF2BP1/2/3 can increase mRNA stability and translational capacity [146].
4 Role of m5C, m1A, m6Am, m7G, and Ψ modification in urological cancers
Recently, there has been a gradual increase in research on m5C and cancer, although the specific mechanism of action of m5C in some cancers remains unclear. In urological cancers, m5C is also in its infancy, and current and future studies will continue to explore the role and molecular mechanisms of m5C. Currently, models of m5C-related regulators for predicting cancer prognosis, including PCa, RCC, and BCa, are constantly being established [147,148,149,150]. It has been reported that the m5C methyltransferase NSUN2 is highly expressed in prostate cancer tissues and is associated with poor patient prognosis. Mechanistically, NSUN2 stabilizes the AR post-transcriptionally through m5C-YBX1-dependent m5C modifications. AR acts as a transcription factor that regulates NSUN2 transcription [151]. In bladder cancer, m5C may play a key role in the hypoxia-glycolytic network. The m5C RNA-binding protein ALYREF stabilizes PKM2 mRNA in an m5C-dependent manner and promotes bladder cancer cell proliferation through PKM2-mediated glycolysis [152].
ALKBH3, a demethylase of m1A, is also a potential diagnostic marker for prostate cancer.ALKBH3 is highly expressed in prostate cancer and correlates with disease progression and prognosis [153, 154]. The study of m1A in urological cancers is still limited to computer models and needs to be explored in more in vivo and in vitro experiments [155]. Functional RNAi screens in human bladder cancer cells and mouse models have identified m6Am methyltransferase PCIF1 as a novel tumor suppressor, the first indication of its role for m6Am in cancer [156]. Studies have shown that m7G modification is significantly involved in the development and progression of urological cancers. The m7G methyltransferase METTL1 is highly upregulated in BCa tissues, and its expression is positively correlated with clinically advanced, high-grade tumors. METTL1 plays an oncogenic role in BCa development and progression. Functional experiments have shown that METTL1 deletion effectively inhibits BCa proliferation, migration, and invasion, both in vivo and in vitro. Mechanistically, METTL1 mediates specific RNA translation by altering the m7G modification of tRNA and inhibiting ribosomal pausing during tRNA-mRNA codon recognition [157]. In addition, METTL1 expression is upregulated in RCC and PCa [158]. m7G studies in urological cancers still need to be validated by more functional experiments. Studies on pseudouridine in urological cancers have revealed the predictive value of Ψ in these cancers. For example, the methyltransferase DKC1 of Ψ is elevated in PCa and is expected to be a novel biomarker for PCa [107, 159].
5 Role of m6A modification in urological cancers
m6A methylation modifications and their molecular functions have been studied in many human diseases, particularly the role of m6A-mediated regulation of gene expression in cancer. Different signaling pathways mediate the translational machinery to fulfill anabolic demands in cancerous tissues. Many studies have also depicted that m6A can play a role in the biology of urological cancers via multiple molecular pathways (see Figs. 3,4,5 and Table 2).
Molecular mechanisms by which m6A modifications regulate the biological functions of PCa. Writer proteins METTL3 and METTL14 are involved in PCa proliferation, invasion, migration, bone metastasis, angiogenesis, and glycolysis. Eraser protein FTO inhibits PCa proliferation, invasion, and migration. Reader proteins YTHDF1/2 and IGF2BP2/3 are involved in PCa proliferation, invasion, migration, and bone metastasis
Molecular mechanisms by which m6A modifications regulate the biological functions of BCa. Writer proteins METTL3 and METTL14 are involved in BCa proliferation, invasion, migration, angiogenesis, cell adhesion and immune escape. Eraser protein FTO is involved in BCa proliferation, invasion, and migration. Reader proteins YTHDF1 and IGF2BP3 are involved in BCa proliferation
Molecular mechanisms by which m6A modifications regulate the biological functions of RCC and TC. Writer proteins METTL3, METTL14, WTAP, and RBM15 are involved in RCC proliferation, invasion, and migration. Eraser proteins FTO and ALKBH5 are involved in RCC proliferation, invasion, migration, and autophagy. Reader proteins IGF2BP1/3 are involved in RCC proliferation and energy metabolism. METTL3 is involved in TC drug resistance
5.1 Role of m6A modification in PCa
5.1.1 The role of m6A writers in PCa
METTL3 was the first identified m6A writer and the only catalytic subunit, and its upregulation in PCa translates into playing important roles in cancer progression. METTL3 expression was upregulated in PCa cell lines, where it knockdown-induced apoptosis in cancer cells [160]. METTL3 upregulation was also associated with poor prognosis in PCa patients, as its expression was upregulated in PCa tissues, especially bone metastases [161, 162]. METTL3 was frequently upregulated in PCa as an upstream cooperating factor of YTHDF2. Further analysis of MeRIP-seq, mRNA-seq, and databases identified LHPP and NKX3-1 as the main targets of YTHDF2, while LHPP and NKX3-1 were found to be tumor suppressors regulating tumor progression by inhibiting AKT phosphorylation [163,164,165,166,167], which was also reported by Cai et al. where METTL3 had elevated levels in PCa cells, promoting its growth by regulating the hedgehog pathway [160]. Similarly, METTL3 can also affect Wnt/β-catenin in the Wnt pathway via m6A methylation of LEF1 mRNA to promote PCa proliferation and migration [168]. METTL3 is involved in PCa metastasis by mediating epithelial-mesenchymal transition by regulating the expression of ARHGDIA migration-associated protein [169]. Among non-coding RNAs, the METTL3 is imperative for DGCR8 to regulate pri-miRNAs in PCa, where experiments have revealed that m6A modification-dependent METTL3 can interact with DGCR8 to enhance the recognition of prior-miR-182 in PCa, thereby promoting the maturation of pri-miRNAs, leading to PCa proliferation, in addition, to mediate m6A modification of KIF3C mRNA, thereby promoting PCa progression [170, 171]. METTL3-mediated m6A-modified lncRNA MALAT1 can lead to PCa proliferation by activating the PI3K/AKT signaling pathway, and METTL3-mediated lncRNA PVT1 was found to regulate the miR-27b-3b/BLM signaling axis [172, 173]. Lang et al. identified a novel molecular mechanism of bone metastasis in which METTL3-mediated m6A modification promotes the upregulation of PCAT6 in an IGFBP2-dependent manner. PACT6 enhances IGF1R mRNA stability through the PACT6-IGF2BP2-IGF1R RNA–protein trimer, thereby upregulating IGF1R expression and promoting bone metastasis and tumor growth in PCa [174]. Glycolysis is the preferred pathway for energy acquisition by cancer cells; however, glycolysis is not a hallmark of primary prostate cancer and plays a critical role only in advanced tumors [175,176,177]. METTL3 enhances SNHG7 stability by regulating m6A modifications of SNHG7 and recruits SRSF1 to regulate c-Myc expression, further promoting glycolysis in PCa cells [178].
Moreover, among other components of methyltransferases, high expression of VIRMA may also be associated with poor prognosis of PCa [179]. It was also discovered that METTL14 promoted PCa proliferation in an m6A-dependent manner by inhibiting the expression of THBS6, an angiogenesis-inhibiting glycoprotein [180].
5.1.2 Role of m6A erasers in PCa
RNA modifications were demonstrated to be reversible with the discovery of FTO and ALKBH5. FTO is commonly downregulated in PCa tissues and cell lines, and patients with lower FTO expression have more advanced tumor stages and higher Gleason scores [181, 182]. Li et al. reported that FTO inhibits PCa progression by downregulating melanocortin receptor 4 (MC4R) expression [183]. Furthermore, downregulation of FTO was associated with a poor prognosis of PCa, and functional experiments demonstrated that FTO depletion promoted PCa proliferation and metastasis in vivo and in vitro. Chloride intracellular channel 4(CLIC4) is a functional target of FTO-mediated m6A modifications. FTO inhibits PCa proliferation and metastasis by reducing CLIC4 mRNA in an m6A-dependent manner [184]. Similarly, a recent study discovered that ALKBH5 expression is downregulated in PCa tissues, inhibiting the growth of PCa cell lines [185]. Overall, studies on m6A erasers in PCa are limited and require further exploration.
5.1.3 Role of m6A readers in PCa
m6A readers also play an important role in PCa progression. YTHDC1 was found to bind and colocalize with oncogene MET adhesin in subnuclear patches and affect PCa proliferation [186]. YTHDC2 expression was upregulated in PCa tissues and cell lines and significantly correlated with PSA levels and Gleason scores, whereas YTHDC2 overexpression promoted proliferation and invasion in PCa cell lines [187]. It was also discovered that YTHDF1/2 was overexpressed in PCa, and PLK1, a critical cell cycle factor, is a direct target of YTHDF1 in PCa cells. Furthermore, ELK1-activated YTHDF1 controls PLK1 translation efficiency in an m6A-dependent manner, enabling activation of the PI3K/AKT signaling pathway, leading to PCa progression. YTHDF1 can also contribute to PCa progression by regulating TRIM44 to promote PCa cell proliferation and migration [188, 189]. YTHDF2 enables PCa progression by mediating the degradation of the tumor suppressors LHPP and NKX3-1 and activating the AKT signaling pathway [167]. YTHDF2 also served as a direct target of miR-495 and miR-493-3p. On the lysine demethylase 5a (KDM5a)/miRNA495/YTHDF2/ m6AMOB3b axis, YTHDF2 recognizes m6A of MOB3b mRNA, inducing MOB3b mRNA degradation and suppresses its expression. The miR-493-3p suppresses YTHDF2 expression, thereby increasing the level of m6A [190, 191]. Therefore, high expression levels of YTHDF2 promote the proliferation, migration, and invasion of PCa cells.
hnRNPA2B1 is highly expressed in CRPC cells, promoting proliferation and leading to a worse prognosis of PCa [192]. IGF2BP2 promotes the upregulation of PCAT6 in an m6A-dependent manner. In addition, PCAT6 enhances IGF1R mRNA stability via the PCAT6/IGF2BP2/IGF1R RNA–protein trimer, thereby upregulating IGF1R expression and promoting PCa bone metastasis and tumor growth [174]. A clinical case study depicted that IGF2BP3 was associated with infiltrative tumor recurrence [193]. IGF2BP3 also binds to cyclic RNA hsa_circ_0003258 in the cytoplasm, enhancing the stability of HDAC4 mRNA, activating the ERK pathway, and triggering EMT to accelerate the transfer of PCa [194].
5.2 Role of m6A modification in BCa
5.2.1 The role of m6A writers in BCa
Several studies have revealed that METTL3, a core component of m6A methyltransferase, is significantly upregulated in BCa and contributes to cancer progression. For instance, Han et al. found that METTL3 may have oncogenic effects in BCa by interacting with DGCR8 and positively regulating the pri-miR222/6 process in an m6A-dependent manner [195]. MEETL3 downregulation significantly reduced BCa proliferation, invasion, and tumorigenicity in vivo. In contrast, overexpression of METTL3 promoted BCa cell growth, mechanistically triggered by METTL3-mediated m6A modification, mediating activation of the AFF4/NF-κb/Myc signaling pathway [196]. Activation of JNK signaling is also associated with increased METTL3 expression in Bca, where knocking down of JNK1 or administration of JNK inhibitors resulted in impairment of c-JUN binding to the METTL3 promoter, thereby reducing the expression of METTL3 and global RNA m6A levels, in addition to JNK signaling to suppress PD-L1 mRNA expression abundance, which revealed that METTL3 could promote BCa immune escape [197]. Xie et al. employed MeRIP and found that the METTL3/YTHDF2 m6A axis directly degrades the mRNAs of tumor suppressors SETD7 and KLF4 and promotes BCa development, whereas METTL3 depletion contributes to the impairment of cancer proliferation and metastasis [198]. The METTL3 is also associated with BCa cell adhesion; reported by Jin et al. that upregulation of the adhesion factor ITGA6 correlated with increased METTL3 expression in human BC tissues, and higher ITGA6 expression in patients was associated with lower survival rates. Mechanistically, m6A is highly enriched in ITGA6 transcripts, and increased m6A methylation of the ITGA6 mRNA 3 promoter promotes translation of ITGA6 mRNA by binding to m6A readers YTHDF1 and YTHDF3 [199]. Similarly, Wang et al. found that removing METTL3 in the bladder uroepithelium attenuated bladder carcinogenesis and tumor angiogenesis. Furthermore, conditional knockdown of METTL3 in BCa stem cell populations inhibits Bca progression. Combining transcriptome sequencing and methylation RNA immunoprecipitation sequencing results, we found that METTL3 deletion reduced the abundance of tyrosine kinase endothelial (TEK) and vascular endothelial growth factor A (VEGF-A) m6A peaks in specific regions. In addition, the deletion of METTL3 reduced the mRNA and protein levels of both TEK and VEGF-A in vitro. Taken together, METTL3-mediated m6A modification is required for the activation of TEK-VEGF-A-mediated tumor progression and angiogenesis [200]. Yang et al. reported that METTL3 and CDCP1 expression was upregulated in BCa tissues, and their expression levels were correlated with BCa progression, in addition to the METTL3-m6A-CDCP1 axis inhibiting chemotransformation and BCa cell growth and progression. This axis synergizes with chemical carcinogens to promote malignant transformation of uroepithelial cells and BCa development [201]. Ying et al. demonstrated that the RCas9-METTL3 system mediates efficient site-specific m6A installation on CDCP1 mRNA, thereby promoting BC progression [202]. Long-term exposure to fine particulate matter (PM2.5) is also associated with various cancers, including Bca, which was reported in a study by Liu et al. stating that PM2.5 exposure was significantly associated with increased levels of m6A modification in Bca patients and bladder cells, with abnormally upregulated METTL3 expression. METTL3 is also involved in PM2.5-induced m6A methylation; enhancing METTL3 expression induces METTL3 promoter hypermethylation and increases the binding affinity of the transcription factor HIF1A mechanistically. Similarly, the BIRC5 was identified as the target gene of METTL3 by m6A sequencing (m6A- seq) and KEGG analysis. The methylated BIRC5 transcripts are subsequently recognized by IGF2BP3, enhancing its mRNA stability. In particular, PM2.5 exposure promoted m6A modification of BIRC5 and its recognition by IGF2BP3. In addition, BIRC5 is involved in BCa proliferation and metastasis, as well as in VEGFA-regulated angiogenesis. It was also revealed that PM2.5 exposure exerts epigenetic regulation on BCa through the HIF1A/METTL3/IGF2BP3/BIRC5/VEGFA axis [203].
In other "writer" studies, Gu et al. found lower expression of METTL4 in BCa, where knocking down METTL4 promoted BCa proliferation, self-renewal, metastasis, and tumor initiation. In contrast, the opposite effects were observed in the case of overexpressed METTL4 [204]. In addition, Teixeira et al. found that METTL14 knockdown disrupted the remaining methyltransferase complex, decreased m6A abundance, and reduced tumor aggressiveness, including decreased cell invasion and migration capacity and increased apoptosis. Furthermore, in vivo, METTL14 knockdown also reduces tumor size [205]. The biological role played by METTL14 in BCa needs to be supported by more studies.
5.2.2 Role of m6A erasers in BCa
Recent studies have identified FTO as a critical factor for the oncogenic effects of BCa. It has been shown that FTO exhibits oncogenic effects in BCa by regulating the expression of cell cycle protein-dependent kinase (CDK6), which is closely related to the cell cycle, and mechanistically promotes cancer cell proliferation and invasion in BCa through the FTO/miR-576/CDK6 pathway [206]. Song et al. found that post-translational deubiquitination of USP18 upregulates FTO protein expression, whereas FTO promotes the occurrence and development of BCa through its demethylase activity on PYCR1 and stabilizes its transcript [207]. In addition, FTO regulates the MALAT/miR-384/MAL2 axis through m6A RNA modification, leading to Bca [208]. Jin et al. displayed that METTL3 and ALKBH5 altered cell adhesion by regulating ITGA6 expression in BC cells, suggesting an oncogenic effect of m6A-modified ITGA6 and its regulatory mechanism on BCa initiation and progression. Simultaneously, the downregulation of ALKBH5 promotes BCa cell proliferation, invasion, and migration [209].
5.2.3 Role of m6A readers in BCa
All three YTHDF proteins in BCa were oncogenic. For instance, YTHDF1/YTHDF3 promotes tumor growth and progression by recognizing m6A-modified ITGA6 mRNA and promoting its translation [199]. In addition, YTHDF1 promotes BCa cell proliferation through the METTL3/YTHDF1-RPN2-PI3K/AKT/mTOR regulatory axis [210]. YTHDF2 promotes tumorigenesis by accelerating the degradation of oncogenes SETD7 mRNA and KLF4 mRNA in BCa [198], in addition to mediating the downregulation of oncogene RIG-I levels through m6A modification, thereby promoting BCa cell proliferation. Xie et al. found that IGF2BP1 binds to circPTPRA in the cytoplasm of BC cells and that ectopic expression of circPTPRA abolishes the promotion of BCa cell growth and metastasis induced by IGF2BP1 [211]. Huang et al. also reported elevated expression of IGF2BP3 in BCa tissues, where its overexpression significantly promoted the cell cycle and BC cell proliferation by activating the JAK/STAT signaling pathway and inhibiting apoptosis [212].
5.3 Role of m6A modification in RCC
5.3.1 The role of m6A writers in RCC
There is now substantial evidence that m6A modifications mediated by different regulatory factors affect RCC progression by inhibiting or promoting its effects. Zhu et al. found that METTL3 expression was significantly higher in RCC tissues than in adjacent normal tissues and that cell viability, migratory capacity, invasive capacity, and in vivo tumor formation were significantly inhibited when METTL3 was depleted [213]. It was observed that METTL3 promotes RCC tumor progression, migration, and tumor spheroid (stem cell-like tumorigenic cell) formation through m6A modification-mediated ABCD1 translation [214]. It has been reported that decreasing MEEL14 reduces m6A modification of BPTF, which enhances mRNA stability and protein expression, leading to glycolytic reprogramming and metabolic remodeling in RCC cells [215]. Similarly, reduced METTL14 was revealed to promote the translation of P2X6 mRNA, activate the ATP-P2X6-Ca2 + -p-ERK1/ERK2-MMP9 signaling pathway, translate into cell migration and invasion, and is detrimental to the prognosis of RCC [216]. It was also demonstrated that enhancement of mucin 15 (MUC15) and m6A modification of histidine-rich glycoprotein (HRG) mRNA using dCas13b-M3M14 fusion protein inhibited the metastasis of kidney cancer, further confirming the role of METTL3 and METTL14 in kidney cancer and providing a potential intervention strategy [217]. The mir-501-3p-CDK2 axis increases WTAP expression in RCC tissues, enhancing the stability and expression of S1PR3 mRNA in an IGF2BPs-dependent manner, thereby driving renal cell carcinogenesis, metastasis and overall poor survival through the regulation of the PI3K/Akt signaling pathway [218,219,220]. Zeng et al. found that RBM15 expression was upregulated in RCC cells and tissues, where EP300/CBP-induced acetylation modification of the RBM15 promoter led to enhanced RBM15 expression and enhanced the expression and stability of CXCL11, thereby promoting macrophage infiltration and M2 polarization, leading to cancer progression in an m6A-dependent manner [221].
5.3.2 The role of m6A erasers in RCC
Demethylases are dysregulated in the development and progression of RCC, and these differences are often associated with metastasis, survival, and poor prognosis. The transcript and protein levels of demethylases, including FTO and ALKBH5, differ across subtypes of renal cancer. A previous study demonstrated that upregulated expression of ALKBH5 regulates AURKB mRNA expression in an m6A-dependent manner, thereby promoting cancer cell proliferation, migration, and invasion, leading to increased RCC volume and poor prognosis [222]. However, dcas13b-ALKBH5-induced demethylation of procollagen lysine and 2-ketoglutarate-5-dioxygenase 2 (PLOD2) mRNA has been shown to play an inverse role [223]. Similarly, FTO has been shown to have opposite roles in RCC. It was found that m6A modification levels are reduced in RCC and are closely associated with autophagy, and silencing FTO impairs RCC growth and metastasis. Mechanistically, SIK2 was identified as a functional target of m6A-mediated autophagy, thus prompting a conserved and important role of FTO in inhibiting autophagy and promoting tumorigenesis through an m6A-IGF2BP2-dependent mechanism [224]. However, the inconsistent roles of FTO and ALKBH5 in RCC are most likely due to the neglect of tumor heterogeneity, different RCC subtypes, or enzyme interaction [225,226,227,228].
5.3.3 The role of m6A readers in RCC
A previous study reported that m6A methylation recognition enzymes are commonly dysregulated in RCC [229]. For example, Wu et al. identified hnRNPC as a potential target biomarker for RCC by examining the in vitro expression level, survival outcome, PPI network, functional enrichment, immune cell infiltration, and single-cell analysis. hnRNP promotes RCC cell proliferation and migration [230]. As known m6A readers, IGF2BPs proteins are upregulated in most cancers and mediate enhanced m6A-modified mRNA stability. Ying et al. found that early EGR2 transcription factors increase IGF2BPs expression in kidney cancer. Similarly, igF2BPs enhance the stability of sphingosine 1-phosphate receptor 3 (S1PR3) mRNA by enhancing the m6A-dependent expression of IGF2BPs, which regulates S1PR3 expression in an m6A-dependent manner and promotes renal tumorigenesis through the PI3K/AKT pathway [220]. IGFBP1 promotes RCC tumor energy metabolism, including glucose uptake, lactate survival, and extracellular acidification rate, by recognizing the m6A modification site on LDHA mRNA and enhancing its mRNA stability, thereby accelerating tumor energy metabolism [231]. IGF2BP3 binds to DDRMR and specifically enhances IGF2BP3 activity against target genes, including the cell cycle kinase CDK4 and three extracellular matrix components (COL6A1, LAMA5, and FN1), in an m6A-dependent manner, thereby stabilizing these genes. Similarly, DDRMR and IGF2BP3 promote the transition of ccRCC cells from G1 to S phase, thereby promoting cell proliferation [232]. In the YTH family, YTHDF2 is associated with immune infiltration and RCC [233, 234].
5.4 Role of m6A modification in TC
METTL3 is an important methylation enzyme that is associated with cancer progression. Luo et al. found that METTL3 is involved in the proliferation, migration, and invasion of TC cells by regulating the expression of EMT-related genes and may also play a role in activating tumor immune responses in TC [235]. METTL3 also regulates the mRNA stability of transcription factor activation enhancer binding protein 2C (TFAP2C) through m6A modification, leading to cisplatin resistance in TC [236]. Knockdown of VIRMA, another methyltransferase, results in disruption of the remaining methyltransferase complex and decreased m6A abundance, resulting in reduced overall invasiveness of TC (reduced cell viability, tumor cell proliferation, migration, and invasion) and increased sensitivity to cisplatin treatment, demonstrated both in vitro and in vivo [237]. The functions of the three different methylesterases of m6A in TC are still scarcely studied, requiring further confirmatory studies.
6 Clinical potential of m6A in urological cancers
The dynamic and reversible roles of RNA modifications in pathology have been recognized. The development of different tumor types is associated with different functions of RNA modifications. With the development of transcriptomics, failure of early diagnosis and poor prognosis due to ineffective treatment remain prevalent in the management of patients with cancer, including urological tumors. Therefore, these regulatory proteins have great potential for early diagnosis, improved prognosis, and treatment of urological cancers as new diagnostic, prognostic, and therapeutic targets.
6.1 m6A-modified regulators as new biomarkers for urological cancers
Various studies have shown a correlation between m6A methylation and cancer initiation and progression. Many recent studies have identified m6A methylation as a diagnostic and prognostic-related biomarker for cancer. For example, Ji et al. found that IGF2BP3, hnRNPA2B1, and METTL14 are significantly associated with PCa prognosis [238]. RCC patients with higher IGFBP3 expression also depict longer metastasis-free and overall survival [239]. Cheng et al. found that WTAP expression was significantly higher in the BC group than in the control group by comparing fresh BCa and normal bladder mucosa specimens, and a significant difference in the risk of disease recurrence was observed between patients with negative and positive WTAP protein expression levels [240]. In addition, METTL3, ALKBH5, IGF2BP3, and FTO levels are closely associated with the prognosis of BC patients [195, 208, 209, 212]. In RCC, the expression levels of ALKBH5 and FTO are associated with shorter overall and cancer-specific survival after nephrectomy and can be used as prognostic biomarkers [226]. Other methylation regulators, including METTL3, WTAP, and IGFBP1/2/3, are also associated with RCC prognosis [241]. Cong et al. screened a database for YTHDF1, RBM15, IGFBP1, ZC3H13, and regulatory factors that play an important role in the prognosis of TC patients [242].
6.2 Potential drug and therapeutic strategies based on m6A-modified regulators
Given that RNA modifications, especially m6A modifications, play an important biological role in various types of cancers, developing targeted drugs based on m6A modifications has become a promising strategy. For example, an inhibitor based on METTL3, STM2457, developed as a treatment option for hematological malignancies, blocks the proliferation and colony formation of MOLM-13 cell lines and promotes apoptosis without affecting normal hematopoietic function. In in vivo studies, STM2457 inhibited the proliferation of acute myeloid leukemia (AML) in patient-derived xenograft models and leukemia mouse models [243]. Cheng et al. developed two potent FTO inhibitors, FB23 and FB23-2, which directly bind to FTO and selectively block its m6A demethylase activity [244]. Subsequently, they developed two other FTO inhibitors, CS1 and CS2, which exhibited strong antitumor effects in various cancers. In leukemia, FTO inhibitors block the FTO/m6A/MYC/CEBPA signaling axis, inhibiting the autologous renewal of tumor stem cells and the expression of the immune checkpoint LILRB4 and immune evasion, thereby enhancing the cytotoxicity of T cells [245]. Many studies have demonstrated that m6A regulators are important in regulating the tumor immune microenvironment. For example, YTHDF2 acts as an m6A reader that isolates m6A-circRNA and plays a vital role in suppressing intrinsic immunity [246]. Han et al. demonstrated that YTHDF1 exerts antitumor effects through m6A methylation in dendritic cells (DCs). Antigen-specific CD1+ T-cell antitumor responses are significantly enhanced in YTHDF1-deficient mice, significantly improving the therapeutic effect of PD-L1 checkpoint blockade [247]. Cheng et al. reported that hnRNPC suppresses PCa tumor immunity by increasing Treg cell activation and suppressing effector CD8 T-cells. Epigenetic alterations can also lead to resistance to chemotherapy and radiotherapy, limiting their efficacy [248], and changes in the expression level of METTL3 made PCa cells resistant to AR antagonists [249]. A newly identified circRNA circ0008399 binds to WTAP and reduces cisplatin sensitivity in BCa by regulating target RNA expression through m6A modification, suggesting the potential therapeutic value of targeting this axis [250]. Chen et al. found that n6-methyladenosine-modified TRAF1 promotes resistance to sunitinib in renal cancer by regulating apoptosis and angiogenesis in a METTL14-dependent manner [251]. In vivo and in vitro assays further demonstrated that the knockdown of VIRMA resulted in an enhanced responsiveness of TC to cisplatin and a significant increase in DNA damage [237].
7 Problems in the clinical application of RNA modification
With the recent development of effective inhibitors against m6A-modified proteins, progress in this area has been demonstrated. However, their use as therapeutic agents remains challenging, mainly due to the lack of consistent and consolidated proof. For example, the oncogenic or pro-carcinogenic nature of the aberrant deposition of m6A in different types of cancer. Although several methylases and Ψ-synthases have been identified that have been found to be aberrantly expressed in cancer, it remains unclear whether they can be effective targets for cancer therapy. In addition, little is known about how the binding of their writers, erasers, and readers affects RNA metabolism and the fate of tumor cells. However, it is undeniable that the availability and selective inhibitors of the 3D structures of most of these regulatory factors have been discovered, suggesting that inhibition of these enzymes is achievable [252]. For example, for the m5C methylases azacytidine and decitabine are cytidine analogs that inhibit any m5C methylase and have been approved for clinical use in hematologic malignancies [253]. However, these inhibitors lack specificity and should be used with caution.
From a clinical perspective, Ψ may have potential as an early biomarker. Large amounts of Ψ have been detected in urine of patients with colon cancer, prostate cancer ovarian cancer, and salivary metabolites of patients with oral squamous cell carcinoma, suggesting that it could be used as a potential biomarker for early cancer diagnosis in non-invasive biopsies [254,255,256,257]. Overall, there are still many problems with the clinical application of RNA modification, but this does not prevent us from affirming the development of small-molecule inhibitors targeting RNA modification sites and RNA modifying enzymes, which will provide a more targeted approach to cancer therapy. We need more relevant studies to validate and further explain the specific mechanisms of RNA methylation in cancer and to resolve some conflicting studies.
8 Discussion
Currently, the biological functions of m6A in regulating urologic cancer mainly focus on proliferation, invasion, migration, angiogenesis, immune escape, and drug resistance [258, 259]. However, tumor metabolic reprogramming, an important cancer feature, has been neglected because of the flexible changes in cancer cell metabolism; it can not only meet the needs of cell growth but also maintain the homeostasis of the tissue environment. Metabolic adaptations are acquired through endogenous and exogenous signaling pathways [260]. Glucose and lipid metabolism are important components of tumor metabolism. Glucose metabolism can be divided into catabolism and anabolism processes. It is well known that energy metabolism is a hallmark of the high invasiveness of cancer cells, including increased glycolytic activity, lactic acid fermentation, and the Warburg effect [259, 261]. The Warburg effect is an important feature of abnormal glucose metabolism in tumors. It can increase glycolysis and glucose uptake and consumption, which causes tumor cells to proliferate differently from normal cells [262].
In urological cancers, specific alterations in many metabolic pathways are also important features [263,264,265]. For example, RCC is essentially a metabolic disease characterized by reprogramming energy metabolism [266]. In particular, metabolic flow through glycolysis is fragmented, and mitochondrial bioenergetics, oxidative enzymes, and lipid metabolism [266,267,268,269,270,271,272]. In this context, the role of m6A RNA methylation as a regulator of cancer cell metabolism in urologic tumors is also a topic of discussion. In prostate cancer, glycolysis is not a hallmark of primary prostate cancer and plays a key role only in advanced cancers [175,176,177]. It has been shown that METTL3 enhances SNHG7 stability by regulating m6A modification of SNHG7 and recruits SRSF1 to regulate c-Myc expression, further promoting glycolysis in PCa cells [178]. Amino acids are produced by proteolysis. The metabolism of amino acids in the body occurs mainly through synthesizing nitrogenous substances, such as proteins and peptides, and the decomposition of amino acids to produce α-ketoacids and CO2 through deamination and transamination [273]. VHL proteins are recognition sites for HIF family substrates, and targeting the HIF family degrades ubiquitin-mediated proteasomes. In RCC, tumor suppressor VHL deletion is an important marker. Inactivation of VHL leads to activation of VEGF and PDGF and targets the downstream glutamine transporter SLC1A5, promoting metabolic reprogramming of VHL-deficient RCC and selectively reducing the growth of VHL-deficient RCC [227]. Mitochondria are the energy factories of the cell, producing the energy currency ATP for the cell by burning glucose, amino acids, and lipids to perform various life activities. There are also many correlations between mitochondrial metabolism and tumorigenesis [274]. Methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) is a mitochondrial enzyme involved in single-carbon metabolism that regulates the HIF-2α transcriptome, thus influencing RCC progression. Although MTHDF2 is still not clearly defined as a methyltransferase of m6A, MTHFD2 expression is significantly elevated in renal cancer cells and regulates the level of m6A methylation. mTHDF2 regulates HIF-2α m6A methylation and promotes HIF-2α expression, thereby facilitating the metabolic reprogramming of tumor cells [275].
Tumorigenesis and development cannot be supported by the tumor microenvironment, which is mainly characterized by hypoxia, metabolic dysregulation, inflammation, and immune infiltration [276, 277]. Evidence shows that m6A mediates biological processes in cancer and stromal cells through splicing, translation, degradation, and export of regulatory factor expression, thus characterizing the TME. m6A also plays an important role in the complex regulatory network of m6A modifications, which in turn affects tumor initiation, progression, and therapeutic responsiveness [278, 279]. For example, RCC is one of the most immune-invasive tumors, and its immune regulation has a profound impact on the prognosis of RCC [280,281,282]. Whether m6A RNA methylation regulates immune infiltration in TME in urologic cancers and its function is also a question worthy of further exploration.
The treatment of urologic cancers still has many pain points and difficulties. For example, the androgen receptor (AR) plays a crucial role in the pathogenesis of prostate cancer and is late in treatment due to the adaptation of PCa cells to low levels of androgens. The AR system remains continuously activated, leading to the inevitable development of destructively resistant prostate cancer. It has been displayed that METTL 3 plays a functional role in this. Roy et al. found that METTL3 expression was higher in AR-expressing PCa cell lines than in AR-negative PCa cell lines, and similar results were obtained at the protein level. This finding suggests a potential interaction between METTL3 and the androgen signaling pathway. Notably, knockdown of METTL3 resulted in increased expression of the AR target gene NKX3.1 and decreased prostate-specific antigen (PSA) expression, suggesting a direct role of METTL3 in AR expression [283]. Knockdown of METTL3 also resulted in the elevation of key regulators, such as KDm1A, which is involved in PCa initiation and progression and regulates AR expression and function [284,285,286]. Therefore, further studies on the effects of METTL3 on the entire androgen signaling pathway are needed. Because of the role that m6A methylation plays an important role in the splicing process [287, 288], future studies could also explore whether METTL3 plays a role in the progression of PCa to CRPC due to the AR splicing process. m6A is expected to serve as a biomarker for prostate cancer diagnosis and clinical intervention.
Overall, RNA modifications are involved in the biological functions of many types of cancer. The multiple functions and mechanisms of m6A modification involved in developing urological cancers require further investigation.
9 Conclusion and prospects
RNA modifications play an important role as key post-transcriptional regulators of gene expression, and the functional network of interactions involves multiple domains such as metabolism, epigenetics, chromatin remodeling, and the immune system. Great breakthroughs have been made in the transcriptome study, including more than 170 chemical modifications of coding and non-coding RNAs; however, most have focused on the biological functions of one or a few RNA modifications. This article describes the common RNA modifications under study; however, other types of RNA modifications remain to be explored.
Compared to other RNA modifications, m6A modifications are the most abundant and well-characterized internal modifications in mRNA, which prompted the desire to fully understand their specific modes of action. Unlike DNA methylation and histone modifications, m6A RNA modifications target almost all transcripts and extensively regulate their processing, stability, and translation levels [35, 119]. Overall, basic and clinical studies have demonstrated that m6A modifications can dynamically and reversibly regulate biological changes in cancer cells. This has provided new therapeutic ideas and directions for treating urological cancer. This paper reviews the role of RNA modifications, especially m6A modifications, on the proliferation, migration, and invasion of urological cancers and summarizes some potential clinical applications of current m6A modifications. Although the study of m6A methylation modification in urological cancers is still at an early stage, it will be significant and valuable to elucidate the mechanism of m6A methylation modification in the development of urological cancers, screen and explore potential targets, and conduct preclinical trials, which will help establish new therapeutic strategies.
As research continues, m6A modifications are becoming more evident. However, it still faces certain limitations, as most studies currently focus on the role played by only a few regulatory factors, ignoring the importance of the overall study and their synergistic role, which requires further research. Second, many studies target the regulation of downstream factors by m6A methylation and exert oncogenic effects, while studies exploring the upstream factors that can lead to abnormal m6A methylation are rare. Third, in the study of m6A modification in urologic cancers, most researchers have focused on the biological functions of proliferation, migration, and invasion. At the same time, little or no exploration has been done on tumor angiogenesis, glycolipid metabolism, and the maintenance of tumor stem cells, which requires further study. Finally, there is a lack of large-scale multicenter clinical trials to verify its feasibility.
Data availability
Not applicable.
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Filippou P, Ferguson JE 3rd, Nielsen ME. Epidemiology of prostate and testicular cancer. Semin Intervent Radiol. 2016;33(3):182–5.
Gray RE, Harris GT. Renal cell carcinoma: diagnosis and management. Am Fam Physician. 2019;99(3):179–84.
King J, Adra N, Einhorn LH. Testicular cancer: biology to bedside. Cancer Res. 2021;81(21):5369–76.
Larroquette M, Peyraud F, Domblides C, et al. Adjuvant therapy in renal cell carcinoma: current knowledges and future perspectives. Cancer Treat Rev. 2021;97: 102207.
Yang L, Zou X, Zou J, Zhang G. Functions of circular RNAs in bladder, prostate and renal cell cancer (Review). Mol Med Rep. 2021;23(5):56.
Gillessen S, Armstrong A, Attard G, et al. Management of patients with advanced prostate cancer: report from the advanced prostate Cancer Consensus Conference 2021. Eur Urol. 2022;82(1):115–41.
Teo MY, Rathkopf DE, Kantoff P. Treatment of advanced prostate cancer. Annu Rev Med. 2019;70:479–99.
Gamat M, McNeel DG. Androgen deprivation and immunotherapy for the treatment of prostate cancer. Endocr Relat Cancer. 2017;24(12):T297–310.
Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28(13):2144–50.
Frampton JE, Keating GM. Bevacizumab: in first-line treatment of advanced and/or metastatic renal cell carcinoma. BioDrugs. 2008;22(2):113–20.
Singh D. Current updates and future perspectives on the management of renal cell carcinoma. Life Sci. 2021;264: 118632.
Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34.
Nadal R, Bellmunt J. Management of metastatic bladder cancer. Cancer Treat Rev. 2019;76:10–21.
Chovanec M, Cheng L. Advances in diagnosis and treatment of testicular cancer. BMJ. 2022;379: e070499.
Nebbioso A, Tambaro FP, Dell’Aversana C, Altucci L. Cancer epigenetics: Moving forward. PLoS Genet. 2018;14(6): e1007362.
Miranda Furtado CL, Dos Santos Luciano MC, Silva Santos RD, Furtado GP, Moraes MO, Pessoa C. Epidrugs: targeting epigenetic marks in cancer treatment. Epigenetics. 2019;14(12):1164–76.
Yao L, Yin H, Hong M, et al. RNA methylation in hematological malignancies and its interactions with other epigenetic modifications. Leukemia. 2021;35(5):1243–57.
Boccaletto P, Stefaniak F, Ray A, et al. MODOMICS: a database of RNA modification pathways. Nucleic Acids Res. 2022;50(1):D231–5.
He L, Li H, Wu A, Peng Y, Shu G, Yin G. Functions of N6-methyladenosine and its role in cancer. Mol Cancer. 2019;18(1):176.
Li M, Tao Z, Zhao Y, et al. 5-methylcytosine RNA methyltransferases and their potential roles in cancer. J Transl Med. 2022;20(1):214.
Li J, Zhang H, Wang H. N(1)-methyladenosine modification in cancer biology: Current status and future perspectives. Comput Struct Biotechnol J. 2022;20:6578–85.
Barros-Silva D, Klavert J, Jenster G, Jerónimo C, Lafontaine D, Martens-Uzunova ES. The role of OncoSnoRNAs and Ribosomal RNA 2’-O-methylation in Cancer. RNA Biol. 2021;18(sup1):61–74.
Xue C, Chu Q, Zheng Q, et al. Role of main RNA modifications in cancer: N(6)-methyladenosine, 5-methylcytosine, and pseudouridine. Signal Transduct Target Ther. 2022;7(1):142.
Luo Y, Yao Y, Wu P, Zi X, Sun N, He J. The potential role of N(7)-methylguanosine (m7G) in cancer. J Hematol Oncol. 2022;15(1):63.
Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71(10):3971–5.
Adams JM, Cory S. Modified nucleosides and bizarre 5’-termini in mouse myeloma mRNA. Nature. 1975;255(5503):28–33.
Dubin DT, Taylor RH. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975;2(10):1653–68.
Perry RP, Kelley DE, Friderici K, Rottman F. The methylated constituents of L cell messenger RNA: evidence for an unusual cluster at the 5’ terminus. Cell. 1975;4(4):387–94.
Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019;74(4):640–50.
Xiang Y, Laurent B, Hsu CH, et al. RNA m(6)A methylation regulates the ultraviolet-induced DNA damage response. Nature. 2017;543(7646):573–6.
Wang YN, Yu CY, Jin HZ. RNA N(6)-methyladenosine modifications and the immune response. J Immunol Res. 2020;2020:6327614.
Davalos V, Blanco S, Esteller M. SnapShot: messenger RNA modifications. Cell. 2018;174(2):498-498.e1.
Frye M, Harada BT, Behm M, He C. RNA modifications modulate gene expression during development. Science. 2018;361(6409):1346–9.
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149(7):1635–46.
Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell. 2015;162(6):1299–308.
Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519(7544):482–5.
Yang Y, Fan X, Mao M, et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27(5):626–41.
Patil DP, Chen CK, Pickering BF, et al. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature. 2016;537(7620):369–73.
Ma JZ, Yang F, Zhou CC, et al. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology. 2017;65(2):529–43.
Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518(7540):560–4.
Yang D, Qiao J, Wang G, et al. N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 2018;46(8):3906–20.
Warda AS, Kretschmer J, Hackert P, et al. Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep. 2017;18(11):2004–14.
Traube FR, Carell T. The chemistries and consequences of DNA and RNA methylation and demethylation. RNA Biol. 2017;14(9):1099–107.
Xie S, Chen W, Chen K, et al. Emerging roles of RNA methylation in gastrointestinal cancers. Cancer Cell Int. 2020;20(1):585.
Motorin Y, Lyko F, Helm M. 5-methylcytosine in RNA: detection, enzymatic formation and biological functions. Nucleic Acids Res. 2010;38(5):1415–30.
Helm M, Motorin Y. Detecting RNA modifications in the epitranscriptome: predict and validate. Nat Rev Genet. 2017;18(5):275–91.
Strobel MC, Abelson J. Effect of intron mutations on processing and function of Saccharomyces cerevisiae SUP53 tRNA in vitro and in vivo. Mol Cell Biol. 1986;6(7):2663–73.
Chen Y, Sierzputowska-Gracz H, Guenther R, Everett K, Agris PF. 5-Methylcytidine is required for cooperative binding of Mg2+ and a conformational transition at the anticodon stem-loop of yeast phenylalanine tRNA. Biochemistry. 1993;32(38):10249–53.
Schaefer M, Pollex T, Hanna K, et al. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24(15):1590–5.
Tuorto F, Liebers R, Musch T, et al. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat Struct Mol Biol. 2012;19(9):900–5.
Chan CT, Pang YL, Deng W, et al. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat Commun. 2012;3:937.
Shanmugam R, Fierer J, Kaiser S, Helm M, Jurkowski TP, Jeltsch A. Cytosine methylation of tRNA-Asp by DNMT2 has a role in translation of proteins containing poly-Asp sequences. Cell Discov. 2015;1:15010.
Galimand M, Schmitt E, Panvert M, et al. Intrinsic resistance to aminoglycosides in Enterococcus faecium is conferred by the 16S rRNA m5C1404-specific methyltransferase EfmM. RNA. 2011;17(2):251–62.
Doi Y, Wachino JI, Arakawa Y. Aminoglycoside Resistance: The Emergence of Acquired 16S Ribosomal RNA Methyltransferases. Infect Dis Clin North Am. 2016;30(2):523–37.
Reid R, Greene PJ, Santi DV. Exposition of a family of RNA m(5)C methyltransferases from searching genomic and proteomic sequences. Nucleic Acids Res. 1999;27(15):3138–45.
Bujnicki JM, Feder M, Ayres CL, Redman KL. Sequence-structure-function studies of tRNA:m5C methyltransferase Trm4p and its relationship to DNA:m5C and RNA:m5U methyltransferases. Nucleic Acids Res. 2004;32(8):2453–63.
Wang W. mRNA methylation by NSUN2 in cell proliferation. Wiley Interdiscip Rev RNA. 2016;7(6):838–42.
Hussain S, Benavente SB, Nascimento E, et al. The nucleolar RNA methyltransferase Misu (NSun2) is required for mitotic spindle stability. J Cell Biol. 2009;186(1):27–40.
Liu Y, Santi DV. m5C RNA and m5C DNA methyl transferases use different cysteine residues as catalysts. Proc Natl Acad Sci U S A. 2000;97(15):8263–5.
Frye M, Watt FM. The RNA methyltransferase Misu (NSun2) mediates Myc-induced proliferation and is upregulated in tumors. Curr Biol. 2006;16(10):971–81.
Hong B, Brockenbrough JS, Wu P, Aris JP. Nop2p is required for pre-rRNA processing and 60S ribosome subunit synthesis in yeast. Mol Cell Biol. 1997;17(1):378–88.
Nakano S, Suzuki T, Kawarada L, Iwata H, Asano K, Suzuki T. NSUN3 methylase initiates 5-formylcytidine biogenesis in human mitochondrial tRNA(Met). Nat Chem Biol. 2016;12(7):546–51.
Metodiev MD, Spåhr H, Loguercio Polosa P, et al. NSUN4 is a dual function mitochondrial protein required for both methylation of 12S rRNA and coordination of mitoribosomal assembly. PLoS Genet. 2014;10(2): e1004110.
Cámara Y, Asin-Cayuela J, Park CB, et al. MTERF4 regulates translation by targeting the methyltransferase NSUN4 to the mammalian mitochondrial ribosome. Cell Metab. 2011;13(5):527–39.
Schosserer M, Minois N, Angerer TB, et al. Methylation of ribosomal RNA by NSUN5 is a conserved mechanism modulating organismal lifespan. Nat Commun. 2015;6:6158.
Haag S, Warda AS, Kretschmer J, Günnigmann MA, Höbartner C, Bohnsack MT. NSUN6 is a human RNA methyltransferase that catalyzes formation of m5C72 in specific tRNAs. RNA. 2015;21(9):1532–43.
Aguilo F, Li S, Balasubramaniyan N, et al. Deposition of 5-Methylcytosine on Enhancer RNAs Enables the Coactivator Function of PGC-1α. Cell Rep. 2016;14(3):479–92.
Tuorto F, Herbst F, Alerasool N, et al. The tRNA methyltransferase Dnmt2 is required for accurate polypeptide synthesis during haematopoiesis. EMBO J. 2015;34(18):2350–62.
Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466(7310):1129–33.
Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502(7472):472–9.
Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol. 2013;14(6):341–56.
Shen H, Ontiveros RJ, Owens MC, et al. TET-mediated 5-methylcytosine oxidation in tRNA promotes translation. J Biol Chem. 2021;296: 100087.
Liu F, Clark W, Luo G, et al. ALKBH1-Mediated tRNA demethylation regulates translation. Cell. 2016;167(3):816-828.e16.
Yang X, Yang Y, Sun BF, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27(5):606–25.
Yang Y, Wang L, Han X, et al. RNA 5-Methylcytosine Facilitates the Maternal-to-Zygotic Transition by Preventing Maternal mRNA Decay. Mol Cell. 2019;75(6):1188-1202.e11.
Lyabin DN, Eliseeva IA, Ovchinnikov LP. YB-1 protein: functions and regulation. Wiley Interdiscip Rev RNA. 2014;5(1):95–110.
Db DUNN. The occurrence of 1-methyladenine in ribonucleic acid. Biochim Biophys Acta. 1961;46:198–200.
RajBhandary UL, Stuart A, Faulkner RD, Chang SH, Khorana HG. Nucleotide sequence studies on yeast phenylalanine sRNA. Cold Spring Harb Symp Quant Biol. 1966;31:425–34.
Peifer C, Sharma S, Watzinger P, Lamberth S, Kötter P, Entian KD. Yeast Rrp8p, a novel methyltransferase responsible for m1A 645 base modification of 25S rRNA. Nucleic Acids Res. 2013;41(2):1151–63.
Sharma S, Watzinger P, Kötter P, Entian KD. Identification of a novel methyltransferase, Bmt2, responsible for the N-1-methyl-adenosine base modification of 25S rRNA in Saccharomyces cerevisiae. Nucleic Acids Res. 2013;41(10):5428–43.
Li X, Xiong X, Wang K, et al. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat Chem Biol. 2016;12(5):311–6.
Li X, Xiong X, Zhang M, et al. Base-Resolution Mapping Reveals Distinct m(1)A methylome in nuclear- and mitochondrial-encoded transcripts. Mol Cell. 2017;68(5):993-1005.e9.
Wang M, Zhu Y, Wang C, et al. Crystal structure of the two-subunit tRNA m(1)A58 methyltransferase TRM6-TRM61 from Saccharomyces cerevisiae. Sci Rep. 2016;6:32562.
Anderson J, Phan L, Hinnebusch AG. The Gcd10p/Gcd14p complex is the essential two-subunit tRNA(1-methyladenosine) methyltransferase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2000;97(10):5173–8.
Vilardo E, Nachbagauer C, Buzet A, Taschner A, Holzmann J, Rossmanith W. A subcomplex of human mitochondrial RNase P is a bifunctional methyltransferase–extensive moonlighting in mitochondrial tRNA biogenesis. Nucleic Acids Res. 2012;40(22):11583–93.
Chujo T, Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. 2012;18(12):2269–76.
Safra M, Sas-Chen A, Nir R, et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551(7679):251–5.
Waku T, Nakajima Y, Yokoyama W, et al. NML-mediated rRNA base methylation links ribosomal subunit formation to cell proliferation in a p53-dependent manner. J Cell Sci. 2016;129(12):2382–93.
Chen Z, Qi M, Shen B, et al. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47(5):2533–45.
Wei J, Liu F, Lu Z, et al. Differential m(6)A, m(6)A(m), and m(1)A Demethylation Mediated by FTO in the Cell Nucleus and Cytoplasm. Mol Cell. 2018;71(6):973-985.e5.
Dai X, Wang T, Gonzalez G, Wang Y. Identification of YTH Domain-Containing Proteins as the Readers for N1-Methyladenosine in RNA. Anal Chem. 2018;90(11):6380–4.
Ayadi L, Galvanin A, Pichot F, Marchand V, Motorin Y. RNA ribose methylation (2’-O-methylation): Occurrence, biosynthesis and biological functions. Biochim Biophys Acta Gene Regul Mech. 2019;1862(3):253–69.
Cesaro B, Tarullo M, Fatica A. Regulation of Gene Expression by m6Am RNA Modification. Int J Mol Sci. 2023;24(3):8.
Akichika S, Hirano S, Shichino Y, et al. Cap-specific terminal N (6)-methylation of RNA by an RNA polymerase II-associated methyltransferase. Science. 2019;363:6423.
Sugita A, Kuruma S, Yanagisawa N, et al. The cap-specific m6A methyltransferase, PCIF1/CAPAM, is dynamically recruited to the gene promoter in a transcription-dependent manner. J Biochem. 2021;170(2):203–13.
Sendinc E, Valle-Garcia D, Dhall A, et al. PCIF1 Catalyzes m6Am mRNA methylation to regulate gene expression. Mol Cell. 2019;75(3):620-630.e9.
Mauer J, Luo X, Blanjoie A, et al. Reversible methylation of m(6)A(m) in the 5’ cap controls mRNA stability. Nature. 2017;541(7637):371–5.
Pandolfini L, Barbieri I, Bannister AJ, et al. METTL1 Promotes let-7 MicroRNA Processing via m7G Methylation. Mol Cell. 2019;74(6):1278-1290.e9.
Kouzarides T, Pandolfini L, Barbieri I, Bannister AJ, Andrews B. Further Evidence Supporting N7-Methylation of Guanosine (m(7)G) in Human MicroRNAs. Mol Cell. 2020;79(2):201–2.
Alexandrov A, Martzen MR, Phizicky EM. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA. 2002;8(10):1253–66.
Trotman JB, Giltmier AJ, Mukherjee C, Schoenberg DR. RNA guanine-7 methyltransferase catalyzes the methylation of cytoplasmically recapped RNAs. Nucleic Acids Res. 2017;45(18):10726–39.
Haag S, Kretschmer J, Bohnsack MT. WBSCR22/Merm1 is required for late nuclear pre-ribosomal RNA processing and mediates N7-methylation of G1639 in human 18S rRNA. RNA. 2015;21(2):180–7.
Li X, Ma S, Yi C. Pseudouridine: the fifth RNA nucleotide with renewed interests. Curr Opin Chem Biol. 2016;33:108–16.
Ge J, Yu YT. RNA pseudouridylation: new insights into an old modification. Trends Biochem Sci. 2013;38(4):210–8.
Kierzek E, Malgowska M, Lisowiec J, Turner DH, Gdaniec Z, Kierzek R. The contribution of pseudouridine to stabilities and structure of RNAs. Nucleic Acids Res. 2014;42(5):3492–501.
Stockert JA, Weil R, Yadav KK, Kyprianou N, Tewari AK. Pseudouridine as a novel biomarker in prostate cancer. Urol Oncol. 2021;39:63–71.
Schwartz S, Bernstein DA, Mumbach MR, et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159(1):148–62.
Rong D, Sun G, Wu F, et al. Epigenetics: Roles and therapeutic implications of non-coding RNA modifications in human cancers. Mol Ther Nucleic Acids. 2021;25:67–82.
Li X, Zhu P, Ma S, et al. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat Chem Biol. 2015;11(8):592–7.
Borchardt EK, Martinez NM, Gilbert WV. Regulation and Function of RNA Pseudouridylation in Human Cells. Annu Rev Genet. 2020;54:309–36.
Penzo M, Montanaro L. Turning Uridines around: Role of rRNA Pseudouridylation in Ribosome Biogenesis and Ribosomal Function. Biomolecules. 2018;8(2):8.
Rintala-Dempsey AC, Kothe U. Eukaryotic stand-alone pseudouridine synthases - RNA modifying enzymes and emerging regulators of gene expression. RNA Biol. 2017;14(9):1185–96.
Yang Y, Isaac C, Wang C, Dragon F, Pogacic V, Meier UT. Conserved composition of mammalian box H/ACA and box C/D small nucleolar ribonucleoprotein particles and their interaction with the common factor Nopp140. Mol Biol Cell. 2000;11(2):567–77.
Kim MS, Kim SS, Yoo NJ, Lee SH. Expressional analysis of NOLA1, NOLA2, NOLA3 and DKC1, the core proteins in H/ACA riboproteins, in gastric and colorectal cancers. Pathology. 2012;44(6):576–7.
Zhang M, Zhao W, Liu S, et al. H/ACA snoRNP gene family as diagnostic and prognostic biomarkers for hepatocellular carcinoma. Pharmgenomics Pers Med. 2021;14:1331–45.
Perry RP, Kelley DE, LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974;82(3):315–31.
Meyer KD, Jaffrey SR. Rethinking m(6)A Readers, Writers, and Erasers. Annu Rev Cell Dev Biol. 2017;33:319–42.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485(7397):201–6.
Geula S, Moshitch-Moshkovitz S, Dominissini D, et al. Stem cells m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347(6225):1002–6.
Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12(8):767–72.
Ke S, Alemu EA, Mertens C, et al. A majority of m6A residues are in the last exons, allowing the potential for 3’ UTR regulation. Genes Dev. 2015;29(19):2037–53.
Huang X, Guo H, Wang L, Yang L, Shao Z, Zhang W. Recent advances in crosstalk between N6-methyladenosine (m6A) modification and circular RNAs in cancer. Mol Ther Nucleic Acids. 2022;27:947–55.
Niu Y, Zhao X, Wu YS, Li MM, Wang XJ, Yang YG. N6-methyl-adenosine (m6A) in RNA: an old modification with a novel epigenetic function. Genomics Proteomics Bioinformatics. 2013;11(1):8–17.
Zhu W, Wang JZ, Wei JF, Lu C. Role of m6A methyltransferase component VIRMA in multiple human cancers (Review). Cancer Cell Int. 2021;21(1):172.
Chen Z, Zhong X, Xia M, Zhong J. The roles and mechanisms of the m6A reader protein YTHDF1 in tumor biology and human diseases. Mol Ther Nucleic Acids. 2021;26:1270–9.
Petri BJ, Klinge CM. m6A readers, writers, erasers, and the m6A epitranscriptome in breast cancer. J Mol Endocrinol. 2023;70(2):78.
Schöller E, Weichmann F, Treiber T, et al. Interactions, localization, and phosphorylation of the m(6)A generating METTL3-METTL14-WTAP complex. RNA. 2018;24(4):499–512.
Wang P, Doxtader KA, Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell. 2016;63(2):306–17.
Satterwhite ER, Mansfield KD. RNA methyltransferase METTL16: Targets and function. Wiley Interdiscip Rev RNA. 2022;13(2): e1681.
Ping XL, Sun BF, Wang L, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24(2):177–89.
Yue Y, Liu J, Cui X, et al. VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov. 2018;4:10.
Wen J, Lv R, Ma H, et al. Zc3h13 Regulates Nuclear RNA m(6)A methylation and mouse embryonic stem cell self-renewal. Mol Cell. 2018;69(6):1028-1038.e6.
Wang JY, Chen LJ, Qiang P. The Potential Role of N6-Methyladenosine (m6A) Demethylase Fat Mass and Obesity-Associated Gene (FTO) in Human Cancers. Onco Targets Ther. 2020;13:12845–56.
Ueda Y, Ooshio I, Fusamae Y, et al. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep. 2017;7:42271.
Huang H, Weng H, Chen J. m(6)A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020;37(3):270–88.
Jonkhout N, Tran J, Smith MA, Schonrock N, Mattick JS, Novoa EM. The RNA modification landscape in human disease. RNA. 2017;23(12):1754–69.
Jiang X, Liu B, Nie Z, et al. The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 2021;6(1):74.
Fang Z, Mei W, Qu C, et al. Role of m6A writers, erasers and readers in cancer. Exp Hematol Oncol. 2022;11(1):45.
Xu C, Wang X, Liu K, et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10(11):927–9.
Shi H, Wang X, Lu Z, et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 2017;27(3):315–28.
Wang X, Lu Z, Gomez A, et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505(7481):117–20.
Xiao W, Adhikari S, Dahal U, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61(4):507–19.
Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. Regulation of m(6)A Transcripts by the 3’→5’ RNA Helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol Cell. 2017;68(2):374-387.e12.
Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45(10):6051–63.
Zhao Z, Meng J, Su R, et al. Epitranscriptomics in liver disease: basic concepts and therapeutic potential. J Hepatol. 2020;73(3):664–79.
Yu G, Bao J, Zhan M, Wang J, Li X, Gu X, Song S, Yang Q, Liu Y, Wang Z, et al. Comprehensive analysis of m5C methylation regulatory genes and tumor microenvironment in prostate cancer. Front Immunol. 2022;13: 914577.
Xu Z, Chen S, Zhang Y, Liu R, Chen M. Roles of m5C RNA modification patterns in biochemical recurrence and tumor microenvironment characterization of prostate adenocarcinoma. Front Immunol. 2022;13: 869759.
Li Z, Wang S, Chen Y, Huang Y, Li T. 5-methylcytosine-related long noncoding RNAS are potential biomarkers to predict overall survival and regulate tumor-immune environment in patients with bladder cancer. Dis Markers. 2022;2022:3117359.
Li L, Tao Z, Zhao Y, Li M, Zheng J, Li Z, Chen X. Prognostic characteristics and immune effects of N(6)-methyladenosine and 5-methylcytosine-related regulatory factors in clear cell renal cell carcinoma. Front Genet. 2022;13: 864383.
Zhu W, Wan F, Xu W, Liu Z, Wang J, Zhang H, Huang S, Ye D. Positive epigenetic regulation loop between AR and NSUN2 promotes prostate cancer progression. Clin Transl Med. 2022;12: e1028.
Wang JZ, Zhu W, Han J, Yang X, Zhou R, Lu HC, Yu H, Yuan WB, Li PC, Tao J, et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun (Lond). 2021;41:560–75.
Hotta K, Sho M, Fujimoto K, Shimada K, Yamato I, Anai S, Harada H, Tsujikawa K, Konishi N, Shinohara N, et al. Clinical significance and therapeutic potential of prostate cancer antigen-1/ALKBH3 in human renal cell carcinoma. Oncol Rep. 2015;34:648–54.
Koike K, Ueda Y, Hase H, Kitae K, Fusamae Y, Masai S, Inagaki T, Saigo Y, Hirasawa S, Nakajima K, et al. anti-tumor effect of AlkB homolog 3 knockdown in hormone- independent prostate cancer cells. Curr Cancer Drug Targets. 2012;12:847–56.
Fu W, Ding J, You X, Li Q, Pei X, Qin G. Four types of RNA modification writers predict the prognosis of prostate cancer. Andrologia. 2022;54: e14552.
Hensel J, Duex JE, Owens C, Dancik GM, Edwards MG, Frierson HF, Theodorescu D. Patient mutation directed shRNA screen uncovers novel bladder tumor growth suppressors. Mol Cancer Res. 2015;13:1306–15.
Ying X, Liu B, Yuan Z, Huang Y, Chen C, Jiang X, Zhang H, Qi D, Yang S, Lin S, et al. METTL1-m(7) G-EGFR/EFEMP1 axis promotes the bladder cancer development. Clin Transl Med. 2021;11: e675.
Campeanu IJ, Jiang Y, Liu L, Pilecki M, Najor A, Cobani E, Manning M, Zhang XM, Yang ZQ. Multi-omics integration of methyltransferase-like protein family reveals clinical outcomes and functional signatures in human cancer. Sci Rep. 2021;11:14784.
Sieron P, Hader C, Hatina J, Engers R, Wlazlinski A, Müller M, Schulz WA. DKC1 overexpression associated with prostate cancer progression. Br J Cancer. 2009;101:1410–6.
Cai J, Yang F, Zhan H, et al. RNA m(6)A methyltransferase METTL3 promotes the growth of prostate cancer by regulating hedgehog pathway. Onco Targets Ther. 2019;12:9143–52.
Yuan Y, Du Y, Wang L, Liu X. The m6A methyltransferase METTL3 promotes the development and progression of prostate carcinoma via mediating MYC methylation. J Cancer. 2020;11(12):3588–95.
Li E, Wei B, Wang X, Kang R. METTL3 enhances cell adhesion through stabilizing integrin β1 mRNA via an m6A-HuR-dependent mechanism in prostatic carcinoma. Am J Cancer Res. 2020;10:1012–25.
Hindupur SK, Colombi M, Fuhs SR, et al. The protein histidine phosphatase LHPP is a tumour suppressor. Nature. 2018;555(7698):678–82.
Zheng J, Dai X, Chen H, Fang C, Chen J, Sun L. Down-regulation of LHPP in cervical cancer influences cell proliferation, metastasis and apoptosis by modulating AKT. Biochem Biophys Res Commun. 2018;503(2):1108–14.
Eide T, Ramberg H, Glackin C, Tindall D, Taskén KA. TWIST1, A novel androgen-regulated gene, is a target for NKX3-1 in prostate cancer cells. Cancer Cell Int. 2013;13(1):4.
Lei Q, Jiao J, Xin L, et al. NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell. 2006;9(5):367–78.
Li J, Xie H, Ying Y, et al. YTHDF2 mediates the mRNA degradation of the tumor suppressors to induce AKT phosphorylation in N6-methyladenosine-dependent way in prostate cancer. Mol Cancer. 2020;19(1):152.
Ma XX, Cao ZG, Zhao SL. m6A methyltransferase METTL3 promotes the progression of prostate cancer via m6A-modified LEF1. Eur Rev Med Pharmacol Sci. 2020;24(7):3565–71.
Chen Y, Pan C, Wang X, et al. Silencing of METTL3 effectively hinders invasion and metastasis of prostate cancer cells. Theranostics. 2021;11(16):7640–57.
Wang D, Wang X, Huang B, et al. METTL3 promotes prostate cancer progression by regulating miR-182 maturation in m6A-dependent manner. Andrologia. 2022;54(7):1581–91.
Ma H, Zhang F, Zhong Q, Hou J. METTL3-mediated m6A modification of KIF3C-mRNA promotes prostate cancer progression and is negatively regulated by miR-320d. Aging (Albany NY). 2021;13(18):22332–44.
Mao Y, Li W, Weng Y, et al. METTL3-Mediated m(6)A Modification of lncRNA MALAT1 Facilitates Prostate Cancer Growth by Activation of PI3K/AKT Signaling. Cell Transplant. 2022;31:9636897221122996.
Chen B, Liu C, Long H, Bai G, Zhu Y, Xu H. N(6)-methyladenosine-induced long non-coding RNA PVT1 regulates the miR-27b-3p/BLM axis to promote prostate cancer progression. Int J Oncol. 2023;62(1):78.
Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z, Du H, Ren D, Dai Y, Peng X. m(6) A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2-mediated IGF1R mRNA stabilization. Clin Transl Med. 2021;11: e426.
Carvalho TM, Cardoso HJ, Figueira MI, Vaz CV, Socorro S. The peculiarities of cancer cell metabolism: A route to metastasization and a target for therapy. Eur J Med Chem. 2019;171:343–63.
Sadeghi RN, Karami-Tehrani F, Salami S. Targeting prostate cancer cell metabolism: impact of hexokinase and CPT-1 enzymes. Tumour Biol. 2015;36:2893–905.
Jadvar H. PET of glucose metabolism and cellular proliferation in prostate cancer. J Nucl Med. 2016;57:25S-29S.
Liu J, Yuan JF, Wang YZ. METTL3-stabilized lncRNA SNHG7 accelerates glycolysis in prostate cancer via SRSF1/c-Myc axis. Exp Cell Res. 2022;416: 113149.
Liu Z, Zhong J, Zeng J, et al. Characterization of the m6A-associated tumor immune microenvironment in prostate cancer to aid immunotherapy. Front Immunol. 2021;12: 735170.
Wang Y, Chen J, Gao WQ, Yang R. METTL14 promotes prostate tumorigenesis by inhibiting THBS1 via an m6A-YTHDF2-dependent mechanism. Cell Death Discov. 2022;8(1):143.
Wu A, Cremaschi P, Wetterskog D, et al. Genome-wide plasma DNA methylation features of metastatic prostate cancer. J Clin Invest. 2020;130(4):1991–2000.
Zhu K, Li Y, Xu Y. The FTO m(6)A demethylase inhibits the invasion and migration of prostate cancer cells by regulating total m(6)A levels. Life Sci. 2021;271: 119180.
Li S, Cao L. Demethyltransferase FTO alpha-ketoglutarate dependent dioxygenase (FTO) regulates the proliferation, migration, invasion and tumor growth of prostate cancer by modulating the expression of melanocortin 4 receptor (MC4R). Bioengineered. 2022;13(3):5598–612.
Zou L, Chen W, Zhou X, Yang T, Luo J, Long Z, Wu J, Lv D, Mao X, Cen S. N6-methyladenosine demethylase FTO suppressed prostate cancer progression by maintaining CLIC4 mRNA stability. Cell Death Discov. 2022;8:184.
Li X, Liu B, Wang S, Li J, Ge X. MiR-141-3p promotes malignant progression in prostate cancer through AlkB homolog 5-mediated m(6)A modification of protein arginine methyltransferase 6. Chin J Physiol. 2023;66(1):43–51.
Luxton HJ, Simpson BS, Mills IG, et al. The Oncogene Metadherin Interacts with the Known Splicing Proteins YTHDC1, Sam68 and T-STAR and Plays a Novel Role in Alternative mRNA Splicing. Cancers (Basel). 2019;11(9):89.
Song J, You G, Yin X, et al. Overexpression of YTHDC2 contributes to the progression of prostate cancer and predicts poor outcomes in patients with prostate cancer. J Biochem Mol Toxicol. 2023;89:e23308.
Li P, Shi Y, Gao D, et al. ELK1-mediated YTHDF1 drives prostate cancer progression by facilitating the translation of Polo-like kinase 1 in an m6A dependent manner. Int J Biol Sci. 2022;18(16):6145–62.
Li W, Chen G, Feng Z, et al. YTHDF1 promotes the proliferation, migration, and invasion of prostate cancer cells by regulating TRIM44. Genes Genomics. 2021;43(12):1413–21.
Du C, Lv C, Feng Y, Yu S. Activation of the KDM5A/miRNA-495/YTHDF2/m6A-MOB3B axis facilitates prostate cancer progression. J Exp Clin Cancer Res. 2020;39(1):223.
Li J, Meng S, Xu M, et al. Downregulation of N(6)-methyladenosine binding YTHDF2 protein mediated by miR-493-3p suppresses prostate cancer by elevating N(6)-methyladenosine levels. Oncotarget. 2018;9(3):3752–64.
Cheng Y, Li L, Qin Z, Li X, Qi F. Identification of castration-resistant prostate cancer-related hub genes using weighted gene co-expression network analysis. J Cell Mol Med. 2020;24(14):8006–17.
Chromecki TF, Cha EK, Pummer K, et al. Prognostic value of insulin-like growth factor II mRNA binding protein 3 in patients treated with radical prostatectomy. BJU Int. 2012;110(1):63–8.
Yu YZ, Lv DJ, Wang C, et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol Cancer. 2022;21(1):12.
Han J, Wang JZ, Yang X, et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18(1):110.
Cheng M, Sheng L, Gao Q, et al. The m(6)A methyltransferase METTL3 promotes bladder cancer progression via AFF4/NF-κB/MYC signaling network. Oncogene. 2019;38(19):3667–80.
Ni Z, Sun P, Zheng J, et al. JNK Signaling Promotes Bladder Cancer Immune Escape by Regulating METTL3-Mediated m6A Modification of PD-L1 mRNA. Cancer Res. 2022;82(9):1789–802.
Xie H, Li J, Ying Y, et al. METTL3/YTHDF2 m(6) A axis promotes tumorigenesis by degrading SETD7 and KLF4 mRNAs in bladder cancer. J Cell Mol Med. 2020;24(7):4092–104.
Jin H, Ying X, Que B, et al. N(6)-methyladenosine modification of ITGA6 mRNA promotes the development and progression of bladder cancer. EBioMedicine. 2019;47:195–207.
Wang G, Dai Y, Li K, et al. Deficiency of Mettl3 in bladder cancer stem cells inhibits bladder cancer progression and angiogenesis. Front Cell Dev Biol. 2021;9: 627706.
Yang F, Jin H, Que B, et al. Dynamic m(6)A mRNA methylation reveals the role of METTL3-m(6)A-CDCP1 signaling axis in chemical carcinogenesis. Oncogene. 2019;38(24):4755–72.
Ying X, Jiang X, Zhang H, et al. Programmable N6-methyladenosine modification of CDCP1 mRNA by RCas9-methyltransferase like 3 conjugates promotes bladder cancer development. Mol Cancer. 2020;19(1):169.
Liu H, Gu J, Huang Z, et al. Fine particulate matter induces METTL3-mediated m(6)A modification of BIRC5 mRNA in bladder cancer. J Hazard Mater. 2022;437: 129310.
Gu C, Wang Z, Zhou N, et al. Mettl14 inhibits bladder TIC self-renewal and bladder tumorigenesis through N(6)-methyladenosine of Notch1. Mol Cancer. 2019;18(1):168.
Guimarães-Teixeira C, Lobo J, Miranda-Gonçalves V, et al. Downregulation of m(6) A writer complex member METTL14 in bladder urothelial carcinoma suppresses tumor aggressiveness. Mol Oncol. 2022;16(9):1841–56.
Zhou G, Yan K, Liu J, Gao L, Jiang X, Fan Y. FTO promotes tumour proliferation in bladder cancer via the FTO/miR-576/CDK6 axis in an m6A-dependent manner. Cell Death Discov. 2021;7(1):329.
Song W, Yang K, Luo J, Gao Z, Gao Y. Dysregulation of USP18/FTO/PYCR1 signaling network promotes bladder cancer development and progression. Aging (Albany NY). 2021;13(3):3909–25.
Tao L, Mu X, Chen H, et al. FTO modifies the m6A level of MALAT and promotes bladder cancer progression. Clin Transl Med. 2021;11(2): e310.
Yu H, Yang X, Tang J, et al. ALKBH5 Inhibited Cell Proliferation and Sensitized Bladder Cancer Cells to Cisplatin by m6A-CK2α-Mediated Glycolysis. Mol Ther Nucleic Acids. 2021;23:27–41.
Zhu J, Tong H, Sun Y, Li T, Yang G, He W. YTHDF1 Promotes Bladder Cancer Cell Proliferation via the METTL3/YTHDF1-RPN2-PI3K/AKT/mTOR Axis. Int J Mol Sci. 2023;24:7895.
Xie F, Huang C, Liu F, et al. CircPTPRA blocks the recognition of RNA N(6)-methyladenosine through interacting with IGF2BP1 to suppress bladder cancer progression. Mol Cancer. 2021;20(1):68.
Huang W, Li Y, Zhang C, et al. IGF2BP3 facilitates cell proliferation and tumorigenesis via modulation of JAK/STAT signalling pathway in human bladder cancer. J Cell Mol Med. 2020;24(23):13949–60.
Zhu D, Liu Y, Chen J, et al. The methyltransferase METTL3 promotes tumorigenesis via mediating HHLA2 mRNA m6A modification in human renal cell carcinoma. J Transl Med. 2022;20(1):298.
Shi Y, Dou Y, Zhang J, et al. The RNA N6-Methyladenosine Methyltransferase METTL3 Promotes the Progression of Kidney Cancer via N6-Methyladenosine-Dependent Translational Enhancement of ABCD1. Front Cell Dev Biol. 2021;9: 737498.
Zhang C, Chen L, Liu Y, Huang J, Liu A, Xu Y, Shen Y, He H, Xu D. Downregulated METTL14 accumulates BPTF that reinforces super-enhancers and distal lung metastasis via glycolytic reprogramming in renal cell carcinoma. Theranostics. 2021;11:3676–93.
Gong D, Zhang J, Chen Y, et al. The m(6)A-suppressed P2RX6 activation promotes renal cancer cells migration and invasion through ATP-induced Ca(2+) influx modulating ERK1/2 phosphorylation and MMP9 signaling pathway. J Exp Clin Cancer Res. 2019;38(1):233.
Gan Y, Li A, Liu J, et al. m(6)A-mRNA Methylation Regulates Gene Expression and Programmable m(6)A Modification of Cellular RNAs With CRISPR-Cas13b in Renal Cell Carcinoma. Front Genet. 2021;12: 795611.
He L, Chen S, Ying Y, et al. MicroRNA-501-3p inhibits the proliferation of kidney cancer cells by targeting WTAP. Cancer Med. 2021;10(20):7222–32.
Tang J, Wang F, Cheng G, et al. Wilms’ tumor 1-associating protein promotes renal cell carcinoma proliferation by regulating CDK2 mRNA stability. J Exp Clin Cancer Res. 2018;37(1):40.
Ying Y, Ma X, Fang J, et al. EGR2-mediated regulation of m(6)A reader IGF2BP proteins drive RCC tumorigenesis and metastasis via enhancing S1PR3 mRNA stabilization. Cell Death Dis. 2021;12(8):750.
Zeng X, Chen K, Li L, et al. Epigenetic activation of RBM15 promotes clear cell renal cell carcinoma growth, metastasis and macrophage infiltration by regulating the m6A modification of CXCL11. Free Radic Biol Med. 2022;184:135–47.
Zhang X, Wang F, Wang Z, et al. ALKBH5 promotes the proliferation of renal cell carcinoma by regulating AURKB expression in an m(6)A-dependent manner. Ann Transl Med. 2020;8(10):646.
Cao C, Ma Q, Huang X, et al. Targeted Demethylation of the PLOD2 mRNA inhibits the proliferation and migration of renal cell carcinoma. Front Mol Biosci. 2021;8: 675683.
Xu Y, Zhou J, Li L, Yang W, Zhang Z, Zhang K, Ma K, Xie H, Zhang Z, Cai L, et al. FTO-mediated autophagy promotes progression of clear cell renal cell carcinoma via regulating SIK2 mRNA stability. Int J Biol Sci. 2022;18:5943–62.
Guimarães-Teixeira C, Barros-Silva D, Lobo J, et al. Deregulation of N6-Methyladenosine RNA Modification and Its Erasers FTO/ALKBH5 among the Main Renal Cell Tumor Subtypes. J Pers Med. 2021;11:10.
Strick A, von Hagen F, Gundert L, et al. The N(6) -methyladenosine (m(6) A) erasers alkylation repair homologue 5 (ALKBH5) and fat mass and obesity-associated protein (FTO) are prognostic biomarkers in patients with clear cell renal carcinoma. BJU Int. 2020;125(4):617–24.
Xiao Y, Thakkar KN, Zhao H, et al. The m(6)A RNA demethylase FTO is a HIF-independent synthetic lethal partner with the VHL tumor suppressor. Proc Natl Acad Sci U S A. 2020;117(35):21441–9.
Zhuang C, Zhuang C, Luo X, et al. N6-methyladenosine demethylase FTO suppresses clear cell renal cell carcinoma through a novel FTO-PGC-1α signalling axis. J Cell Mol Med. 2019;23(3):2163–73.
von Hagen F, Gundert L, Strick A, et al. N(6) -Methyladenosine (m(6) A) readers are dysregulated in renal cell carcinoma. Mol Carcinog. 2021;60(5):354–62.
Wu J, Wei Y, Miao C, Wang S, Wang X, Wang Z. Essential m(6)A methylation regulator HNRNPC serves as a targetable biomarker for papillary renal cell carcinoma. J Oncol. 2022;2022:9411692.
Yuan B, Zhou J. N(6)-methyladenosine (m(6)A) reader IGF2BP1 facilitates clear-cell renal cell carcinoma aerobic glycolysis. PeerJ. 2023;11: e14591.
Gu Y, Niu S, Wang Y, et al. DMDRMR-Mediated Regulation of m(6)A-Modified CDK4 by m(6)A Reader IGF2BP3 Drives ccRCC Progression. Cancer Res. 2021;81(4):923–34.
Su G, Liu T, Han X, et al. YTHDF2 is a potential biomarker and associated with immune infiltration in kidney renal clear cell carcinoma. Front Pharmacol. 2021;12: 709548.
Mu Z, Dong D, Sun M, Li L, Wei N, Hu B. Prognostic Value of YTHDF2 in clear cell renal cell carcinoma. Front Oncol. 2020;10:1566.
Luo Y, Sun Y, Li L, Mao Y. METTL3 may regulate testicular germ cell tumors through EMT and immune pathways. Cell Transplant. 2020;29:963689720946653.
Wei J, Yin Y, Zhou J, et al. METTL3 potentiates resistance to cisplatin through m(6) A modification of TFAP2C in seminoma. J Cell Mol Med. 2020;24(19):11366–80.
Miranda-Gonçalves V, Lobo J, Guimarães-Teixeira C, et al. The component of the m(6)A writer complex VIRMA is implicated in aggressive tumor phenotype, DNA damage response and cisplatin resistance in germ cell tumors. J Exp Clin Cancer Res. 2021;40(1):268.
Ji G, Huang C, He S, et al. Comprehensive analysis of m6A regulators prognostic value in prostate cancer. Aging (Albany NY). 2020;12(14):14863–84.
Jiang Z, Chu PG, Woda BA, et al. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol. 2006;7(7):556–64.
Chen L, Wang X. Relationship between the genetic expression of WTAP and bladder cancer and patient prognosis. Oncol Lett. 2018;16(6):6966–70.
Zheng Z, Mao S, Guo Y, et al. N6-methyladenosine RNA methylation regulators participate in malignant progression and have prognostic value in clear cell renal cell carcinoma. Oncol Rep. 2020;43(5):1591–605.
Cong R, Ji C, Zhang J, et al. m6A RNA methylation regulators play an important role in the prognosis of patients with testicular germ cell tumor. Transl Androl Urol. 2021;10(2):662–79.
Yankova E, Blackaby W, Albertella M, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593(7860):597–601.
Huang Y, Su R, Sheng Y, et al. Small-Molecule Targeting of Oncogenic FTO Demethylase in Acute Myeloid Leukemia. Cancer Cell. 2019;35(4):677-691.e10.
Su R, Dong L, Li Y, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38(1):79-96.e11.
Zhang L, Hou C, Chen C, et al. The role of N(6)-methyladenosine (m(6)A) modification in the regulation of circRNAs. Mol Cancer. 2020;19(1):105.
Han D, Liu J, Chen C, et al. Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature. 2019;566(7743):270–4.
Slade D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020;34(5–6):360–94.
Cotter KA, Gallon J, Uebersax N, et al. Mapping of m(6)A and its regulatory targets in prostate cancer reveals a METTL3-low induction of therapy resistance. Mol Cancer Res. 2021;19(8):1398–411.
Wei W, Sun J, Zhang H, et al. Circ0008399 interaction with WTAP promotes assembly and activity of the m(6)A methyltransferase complex and promotes cisplatin resistance in bladder cancer. Cancer Res. 2021;81(24):6142–56.
Chen Y, Lu Z, Qi C, et al. N(6)-methyladenosine-modified TRAF1 promotes sunitinib resistance by regulating apoptosis and angiogenesis in a METTL14-dependent manner in renal cell carcinoma. Mol Cancer. 2022;21(1):111.
Boriack-Sjodin PA, Ribich S, Copeland RA. RNA-modifying proteins as anticancer drug targets. Nat Rev Drug Discov. 2018;17(6):435–53.
Zhou Z, Li HQ, Liu F. DNA methyltransferase inhibitors and their therapeutic potential. Curr Top Med Chem. 2018;18(28):2448–57.
Stockert JA, Gupta A, Herzog B, Yadav SS, Tewari AK, Yadav KK. Predictive value of pseudouridine in prostate cancer. Am J Clin Exp Urol. 2019;7(4):262–72.
Jiang T, Lin Y, Yin H, Wang S, Sun Q, Zhang P, et al. Correlation analysis of urine metabolites and clinical staging in patients with ovarian cancer. Int J Clin Exp Med. 2015;8(10):18165–71.
Krstulja A, Lettieri S, Hall AJ, Roy V, Favetta P, Agrofoglio LA. Tailor-made molecularly imprinted polymer for selective recognition of the urinary tumor marker pseudouridine. Macromol Biosci. 2017;17(12):67.
Sridharan G, Ramani P, Patankar S, Vijayaraghavan R. Evaluation of salivary metabolomics in oral leukoplakia and oral squamous cell carcinoma. J Oral Pathol Med. 2019;48(4):299–306.
Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64.
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, Yi P, Tang L, Pan Q, Rao S, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20:28.
Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells. Drug Resist Updat. 2018;38:1–11.
Lasorsa F, di Meo NA, Rutigliano M, Ferro M, Terracciano D, Tataru OS, Battaglia M, Ditonno P, Lucarelli G. Emerging hallmarks of metabolic reprogramming in prostate cancer. Int J Mol Sci. 2023;24:78.
di Meo NA, Lasorsa F, Rutigliano M, Loizzo D, Ferro M, Stella A, Bizzoca C, Vincenti L, Pandolfo SD, Autorino R, et al. Renal cell carcinoma as a metabolic disease: an update on main pathways, potential biomarkers, and therapeutic targets. Int J Mol Sci. 2022;23:7.
di Meo NA, Loizzo D, Pandolfo SD, Autorino R, Ferro M, Porta C, Stella A, Bizzoca C, Vincenti L, Crocetto F, et al. Metabolomic approaches for detection and identification of biomarkers and altered pathways in bladder cancer. Int J Mol Sci. 2022;23:865.
De Marco S, Torsello B, Minutiello E, Morabito I, Grasselli C, Bombelli S, Zucchini N, Lucarelli G, Strada G, Perego RA, et al. The cross-talk between Abl2 tyrosine kinase and TGFβ1 signalling modulates the invasion of clear cell Renal Cell Carcinoma cells. FEBS Lett. 2023;597:1098–113.
Ragone R, Sallustio F, Piccinonna S, Rutigliano M, Vanessa G, Palazzo S, Lucarelli G, Ditonno P, Battaglia M, Fanizzi FP, et al. Renal cell carcinoma: a study through NMR-based metabolomics combined with transcriptomics. Diseases. 2016;4:6.
Lucarelli G, Galleggiante V, Rutigliano M, Sanguedolce F, Cagiano S, Bufo P, Lastilla G, Maiorano E, Ribatti D, Giglio A, et al. Metabolomic profile of glycolysis and the pentose phosphate pathway identifies the central role of glucose-6-phosphate dehydrogenase in clear cell-renal cell carcinoma. Oncotarget. 2015;6:13371–86.
Lucarelli G, Rutigliano M, Sallustio F, Ribatti D, Giglio A, Lepore Signorile M, Grossi V, Sanese P, Napoli A, Maiorano E, et al. Integrated multi-omics characterization reveals a distinctive metabolic signature and the role of NDUFA4L2 in promoting angiogenesis, chemoresistance, and mitochondrial dysfunction in clear cell renal cell carcinoma. Aging. 2018;10:3957–85.
Bombelli S, Torsello B, De Marco S, Lucarelli G, Cifola I, Grasselli C, Strada G, Bovo G, Perego RA, Bianchi C. 36-kDa Annexin A3 isoform negatively modulates lipid storage in clear cell renal cell carcinoma cells. Am J Pathol. 2020;190:2317–26.
Bianchi C, Meregalli C, Bombelli S, Di Stefano V, Salerno F, Torsello B, De Marco S, Bovo G, Cifola I, Mangano E, et al. The glucose and lipid metabolism reprogramming is grade-dependent in clear cell renal cell carcinoma primary cultures and is targetable to modulate cell viability and proliferation. Oncotarget. 2017;8:113502–15.
Lucarelli G, Rutigliano M, Loizzo D, di Meo NA, Lasorsa F, Mastropasqua M, Maiorano E, Bizzoca C, Vincenti L, Battaglia M, et al. MUC1 Tissue Expression and Its Soluble Form CA15–3 identify a clear cell renal cell carcinoma with distinct metabolic profile and poor clinical outcome. Int J Mol Sci. 2022;23:78.
Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. 2017;3:169–80.
Boese AC, Kang S. Mitochondrial metabolism-mediated redox regulation in cancer progression. Redox Biol. 2021;42: 101870.
Green NH, Galvan DL, Badal SS, Chang BH, LeBleu VS, Long J, Jonasch E, Danesh FR. MTHFD2 links RNA methylation to metabolic reprogramming in renal cell carcinoma. Oncogene. 2019;38:6211–25.
Vuong L, Kotecha RR, Voss MH, Hakimi AA. Tumor microenvironment dynamics in clear-cell renal cell carcinoma. Cancer Discov. 2019;9:1349–57.
Gu Y, Wu X, Zhang J, Fang Y, Pan Y, Shu Y, Ma P. The evolving landscape of N(6)-methyladenosine modification in the tumor microenvironment. Mol Ther. 2021;29:1703–15.
Zhang F, Liu H, Duan M, Wang G, Zhang Z, Wang Y, Qian Y, Yang Z, Jiang X. Crosstalk among m(6)A RNA methylation, hypoxia and metabolic reprogramming in TME: from immunosuppressive microenvironment to clinical application. J Hematol Oncol. 2022;15:84.
Si C, Chen C, Guo Y, Kang Q, Sun Z. Effect, Mechanism, and Applications of Coding/Non-coding RNA m6A Modification in Tumor Microenvironment. Front Cell Dev Biol. 2021;9: 711815.
Tamma R, Rutigliano M, Lucarelli G, Annese T, Ruggieri S, Cascardi E, Napoli A, Battaglia M, Ribatti D. Microvascular density, macrophages, and mast cells in human clear cell renal carcinoma with and without bevacizumab treatment. Urol Oncol. 2019;37(355):e11-355.e19.
Netti GS, Lucarelli G, Spadaccino F, Castellano G, Gigante M, Divella C, Rocchetti MT, Rascio F, Mancini V, Stallone G, et al. PTX3 modulates the immunoflogosis in tumor microenvironment and is a prognostic factor for patients with clear cell renal cell carcinoma. Aging. 2020;12:7585–602.
Lucarelli G, Rutigliano M, Ferro M, Giglio A, Intini A, Triggiano F, Palazzo S, Gigante M, Castellano G, Ranieri E, et al. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol Oncol. 2017;35(461):e15-461.e27.
Lothion-Roy J, Haigh DB, Harris AE, Metzler VM, Alsaleem M, Toss MS, Kariri Y, Ntekim A, Robinson BD, Khani F, et al. Clinical and molecular significance of the RNA m(6)A methyltransferase complex in prostate cancer. Front Genet. 2022;13:1096071.
Cai C, He HH, Chen S, Coleman I, Wang H, Fang Z, Chen S, Nelson PS, Liu XS, Brown M, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20:457–71.
Kashyap V, Ahmad S, Nilsson EM, Helczynski L, Kenna S, Persson JL, Gudas LJ, Mongan NP. The lysine specific demethylase-1 (LSD1/KDm1A) regulates VEGF-A expression in prostate cancer. Mol Oncol. 2013;7:555–66.
Cai C, He HH, Gao S, Chen S, Yu Z, Gao Y, Chen S, Chen MW, Zhang J, Ahmed M, et al. Lysine-specific demethylase 1 has dual functions as a major regulator of androgen receptor transcriptional activity. Cell Rep. 2014;9:1618–27.
Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, Kreim N, Andrade-Navarro MA, Poeck B, Helm M, et al. m(6)A modulates neuronal functions and sex determination in Drosophila. Nature. 2016;540:242–7.
Haussmann IU, Bodi Z, Sanchez-Moran E, Mongan NP, Archer N, Fray RG, Soller M. m(6)A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature. 2016;540:301–4.
Funding
This work was supported by the Jiangxi Province 2021 Postgraduate Innovation Special Funds Project (Grant No. YC2023-S949).
Author information
Authors and Affiliations
Contributions
GZ conceived the manuscript, XZ searched publications and draft the manuscript. HZ, YZ and CL edited tables and figures. XZ, GZ and JZ reviewed the manuscript and polished the grammar. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, X., Zhu, H., Luo, C. et al. The role of RNA modification in urological cancers: mechanisms and clinical potential. Discov Onc 14, 235 (2023). https://doi.org/10.1007/s12672-023-00843-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-023-00843-8