Abstract
Background
Lung cancer is the leading cause of cancer-related mortality worldwide. Sarcopenia, defined as the loss of muscle mass and function, is known to cause adverse health outcomes. The purpose of this umbrella review was to integrate published systematic reviews and meta-analyses exploring sarcopenia and lung cancer to provide comprehensive knowledge on their relationship.
Methods
Eligible studies were searched from scientific databases until June 28, 2022. Critical appraisal was performed using A Measurement Tool to Assess Systematic Reviews (AMSTAR) 2. The impact of sarcopenia on the pathophysiology, prevalence, and prognosis of lung cancer is summarized at the level of systematic reviews or meta-analyses.
Results
Fourteen reviews and meta-analyses were conducted. The methodological quality was high for one review, low for nine, and critically low for four. The most common standard for diagnosing sarcopenia in the lung cancer population is computed tomography (CT) to measure the skeletal muscle index at the third lumbar vertebra (L3). Sarcopenia was highly prevalent among patients with lung cancer, with a pooled prevalence ranging from 42.8% to 45.0%. The association between sarcopenia and increased postoperative complications and decreased disease control rates with immune checkpoint inhibitors has been demonstrated. Mortality was significantly higher in sarcopenic patients than in non-sarcopenic patients with lung cancer, regardless of the stage of disease or type of treatment.
Conclusions
Sarcopenia is a poor prognostic factor for lung cancer. Future studies are necessary to clarify the pathophysiology of sarcopenia and develop effective interventions for sarcopenia in patients with lung cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Lung cancer is a common and unfavorable type of malignancy [1]. Its incidence is on the rise globally, with more than two million estimated new cases per year [1]. The age-standardized cumulative lifetime risk is 3.80% for men and 1.77% for women, making it the second most prevalent cancer in both sexes [2, 3]. Surgical excision, chemotherapy, and radiotherapy have been the traditional cornerstones in the treatment, followed by targeted therapy and immunotherapy. The improved survival in industrialized countries is attributed to decline in tobacco smoking, early detection via low-dose chest tomography, and easy access to the state-of-the-art treatment modalities [3]. Despite substantial efforts and advances, the latest 5-year survival rate (from 2010 to 2016) for lung cancer in the United States of America is 20.5% [4].
Tumor/node/metastasis (TNM) staging based on tumor size, local invasion, and distant spread is the prevailing framework for estimating life expectancy in the cancer population [5]. However, the utility of the TNM system is limited in advanced cancer and in patients receiving targeted therapy and immunotherapy. The functional status represented by the Eastern Cooperative Oncology Group (ECOG) Performance Status Scale is of independent prognostic value in lung cancer. However, its clinical value is limited by its subjective assessment [6]. Weight loss at the initial diagnosis was independently associated with poor outcomes in patients with non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) [7]. Further, patients with NSCLC and weight loss are less responsive to chemotherapy and have an increased withdrawal rate [8]. Therefore, numerous ongoing studies aim to identify more reliable prognostic indicators other than weight loss.
Sarcopenia is a skeletal muscle disorder characterized by progressive generalized loss of muscle mass and function [9, 10]. In case of low muscle strength, sarcopenia can be confirmed by measuring the muscle quantity and quality. Although it was first introduced as a geriatric disease, the condition is not exclusive to older adults and can accompany many diseases. Its associations with cardiac disease, respiratory disease, cognitive impairment, and musculoskeletal disorders have also been observed [11, 12]. Sarcopenia is a pressing clinical issue because it poses increased risks for falls, fractures, functional impairment, hospitalizations, and mortality, and creates hefty healthcare burdens [11, 13]. Accordingly, there has been great interest in the impact of sarcopenia on lung cancer, with several systematic reviews and meta-analyses published to explore the relationship between them. This umbrella review aimed to compile evidence from these systematic reviews and meta-analyses to evaluate the existing information on the interplay between sarcopenia and lung cancer.
2 Methods
2.1 Protocol registration
We conducted an umbrella review according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [14]. The protocol was registered at Inplasy.com with the number INPLASY202270050.
2.2 Search strategy
PubMed, Embase, Web of Science, and Cochrane databases were systematically searched from their inception to June 2022 for articles assessing the relationship between sarcopenia and lung cancer. The following search terms were used: (“cancer” OR “lung cancer” OR “lung neoplasm” OR “lung tumor”) AND (“sarcopenia” OR “skeletal muscle” OR “muscle loss” OR “nutrition”) AND (“systematic review” OR “meta-analysis”). No language restrictions were applied. The gray literature was explored using Google Scholar. Furthermore, the reference lists of eligible articles were manually searched for additional relevant studies. The complete search strategy is presented in the Additional file 1.
2.3 Inclusion and exclusion criteria
Articles were included if they (1) were systematic reviews or meta-analyses and (2) investigated the prevalence, pathophysiology, prognostic capability, or management of sarcopenia in patients with lung cancer. We excluded articles that (1) failed to complete a systematic literature search, (2) did not incorporate participants with primary pulmonary malignancy, (3) analyzed tumors from multiple sites but did not focus on or did not perform a subgroup analysis on lung cancer, and (4) only reported nutritional assessment without defining whether the patients were sarcopenic. Scoping reviews, narrative reviews, review protocols, and conference abstracts were excluded.
2.4 Article selection and data extraction
The titles and abstracts of the papers retrieved from the initial database search were independently screened by two authors (T.-Y. L. and W.-T.W.). The full texts of each potential piece were obtained and reviewed for data extraction. The collected information encompassed the following: first author, country/year of publication, number of included studies/participants, aim of the review, diagnostic criteria for sarcopenia, qualitative outcomes, results from quantitative analyses (effect size, effect model, 95% confidence interval [CI], p value, and I2), and major findings. Any disagreement regarding study selection or data extraction was settled by the corresponding author.
2.5 Quality assessment
Two authors (T.-Y. L. and W.-T.W.) separately performed the critical appraisal of included reviews using A Measurement Tool to Assess Systematic Reviews (AMSTAR) 2, and a consensus was reached after discussion [15]. AMSTAR2, a methodological quality evaluation tool, comprises 16 items, seven of which are of particular importance, i.e., the presence of a precedent protocol, comprehensive literature search, written inclusion/exclusion criteria, risk of bias assessment, appropriate statistical method, sufficient data interpretation, and publication bias consideration. After scoring yes, partial yes, or no for each item, the overall confidence of the systematic review or meta-analysis was graded as high, moderate, low, or critically low.
2.6 Data synthesis
The results of this umbrella review are presented at the systematic review or meta-analysis level. We addressed the similarities and differences in the population, criteria for sarcopenia, and relevant outcomes to gain a complete understanding of the association between sarcopenia and lung cancer. Details of the studies included in each of the eligible reviews are outlined in the Additional file 1.
3 Results
3.1 Literature search
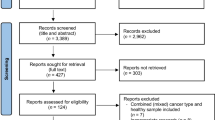
Of the 1797 records generated from the original database search, 1764 were removed for being duplicates or non-relevant literature after title and abstract screening. Full texts were screened for the remaining 33 articles; 15 articles were excluded wherein patients with lung cancer were not regarded as a subgroup during analysis and four for describing nutritional status without focusing on sarcopenia. Finally, 14 reviews [16,17,18,19,20,21,22,23,24,25,26,27,28,29] fulfilled all eligibility criteria and were included in our umbrella review (Fig. 1).
3.2 Study characteristics
Nearly all reviews (except for one [16]) were published after 2019. The research team of eight [18, 20,21,22,23,24,25, 27] reviews was based in Asian countries, five [16, 17, 26, 28, 29] in European countries, and one [19] in the United States of America. Eight [16,17,18,19,20,21, 24, 29] reviews focused on malignancies of pulmonary origin, with four [18, 21, 24, 29] solely examining NSCLC and four [16, 17, 19, 20] encompassing all lung cancers. The remaining six [22, 23, 25,26,27,28] reviews incorporated tumors from multiple locations and dedicated a portion to lung cancer. The majority of reviews revealed the impact of sarcopenia on short-and long-term outcomes in the lung cancer population. Two [16, 29] focused on the broad scope of nutritional status evaluation, and two [26, 28] were centered on the prevalence of sarcopenia. Meta-analyses were conducted in eleven publications [17,18,19,20,21,22,23,24,25, 27, 28]. The characteristics of the included reviews are outlined in Table 1.
3.3 Methodological quality of the included studies
According to the critical appraisal using AMSTAR2, one review [20] had high, nine had low [17, 18, 21,22,23, 25, 27,28,29], and four [16, 17, 19, 24, 26] had critically low evidence quality. Common methodological problems were lack of prior protocol registration (only registered in two reviews [27, 29]), not employing a comprehensive search strategy (three reviews [19, 24, 26] used only PubMed for literature search and 12 [16,17,18,19, 22,23,24,25,26,27,28,29] did not consider trial registries), and not providing sufficient information on the excluded studies (only recorded in three reviews [16, 20, 25]). Seven reviews [18, 19, 22,23,24, 26, 28] did not elaborate on duplicate study selection, and seven [16, 17, 22, 23, 26,27,28] did not show independent data extraction by two authors. The checklist is presented in Table 2.
3.4 Diagnosis and prevalence of sarcopenia in lung cancer
In a pioneer review by Collins et al. [16], sarcopenia was defined by a handful of measuring techniques, including dual-energy x-ray absorptiometry (DEXA), bioelectrical impedance analysis (BIA), computed tomography (CT), upper arm dimensions, grip strength, and skinfold thickness. More recently, CT has become the dominant tool for confirming the diagnosis (Table 3). Skeletal muscle index (SMI, cm2/m2) is calculated by dividing the cumulative skeletal muscle area (SMA, cm2) on a transverse CT slice by the square of the patient’s height. Psoas muscle index (PMI) is calculated using the following formula: total psoas muscle area (cm2) / height (m2). Skeletal muscle density (HU) is another indicator of body composition on CT images, reflecting the intramuscular adipose tissue infiltration or muscle quality. The systematic review by McGovern et al. [26] revealed that the most popular diagnostic thresholds for sarcopenia were derived from large-population studies by Prado et al. [30] (n = 250) and Martin et al. [31] (n = 1473).
Four reviews [19, 20, 26, 28] investigated the prevalence of sarcopenia in patients with lung cancer. Yang et al. [20] recruited 1810 patients from 13 studies and concluded that the pooled prevalence was 45% (95% CI: 32–57%). Meanwhile, the prevalence was 43% (95% CI: 32–54%) in patients with NSCLC and 52% (95% CI: 32–57%) in patients with SCLC. Male patients were more sarcopenic than female patients (53.4% vs. 46.6% for NSCLC and 67.8% vs. 32.2% for SCLC). A review by Nishimura et al. [19] showed similar results, with an overall prevalence of 42.8%. McGovern et al. [26] assembled several studies on CT-assessed muscle mass in the cancer population, among which nine [32,33,34,35,36,37,38,39,40] measured SMI in patients with lung cancer and reported that the median percentage of patients with low SMI was 49.5%. Considering the extent of tumor growth, individuals with non-curative diseases (stage IV, unresectable, and metastatic lung cancer) suffered more frequently from muscle depletion than those with curative diseases (stage I–III lung cancer). The median percentage of sarcopenic patients was 50.3% in the former group compared to 40.2% in the latter group. Likewise, Surov et al. [28] revealed 44.0% regarding the summarized prevalence of sarcopenia from 16 studies, with 36.0% in curative and 51.1% in palliative settings.
3.5 Pathophysiology and treatment of sarcopenia in lung cancer
Systematic reviews by Collins et al. [16] and Nishimura et al. [19] reported concurrent loss of body weight and muscle mass in patients with lung cancer. No significant difference in preoperative serum albumin was noted between the sarcopenic and non-sarcopenic groups [19]. Besides, carcinoembryonic antigen was associated with preoperative sarcopenia in a few, but not all studies, according to the review by Nishimura et al. [19]. Herein, there was no robust evidence on how changes in protein metabolism or genetic polymorphism contributed to the development of muscle mass loss in the lung cancer population [16].
Among the epidemiological variables, Kawaguchi et al. [24] reported a significant association between sarcopenia and smoking habits in three [41,42,43] out of five studies [33, 41,42,43,44]. Nishumura et al. [19] observed that aging was significantly associated with sarcopenia in half of the recruited studies while there was no significant association between sarcopenia and pathologic staging of lung cancer.
Regarding epidemiological variables, Kawaguchi et al. [24] discovered three [41,42,43] out of five [33, 41,42,43,44] studies reporting a significant association between sarcopenia and smoking habits. Nishumura et al. [19]’s review revealed that aging was significantly associated with sarcopenia in half of the selected studies, while there was no significant association between sarcopenia and the pathologic staging of lung cancer.
The performance status was not related to preoperative sarcopenia in the review by Nishumura et al. [19]. In contrast, Collins et al. [16] reported that patients with cachexia had a reduced walking distance and quadriceps strength. There are incoherent findings about the association between forced expiratory volume and preoperative sarcopenia [19]. The effect of nutritional supplements (such as fish oil, protein supplement, and adenosine-5'-triphosphate infusion) on slowing/reversing muscle loss or on improving survival in patients with lung cancer has been contradictory [16].
3.6 The impact of sarcopenia on the prognosis of lung cancer
The prognostic value of sarcopenia in patients with lung cancer was fundamental in the majority of the included reviews. Four reviews [17, 20, 22, 29] incorporated diverse treatment options, such as surgery, chemotherapy, immunotherapy, radiotherapy, or palliative care. Three reviews [18, 19, 24] emphasized on the postoperative outcomes and four [21, 23, 25, 27] focused on immunotherapy.
3.6.1 Postoperative complication rate
The postoperative complication rate was increased in patients with sarcopenia with an odds ratio (OR) of 2.51 (95% CI: 1.55–4.08) in the meta-analysis by Nishumura et al. [19] (involving NSCLC, SCLC, and metastatic disease to the lung) and 1.86 (95% CI: 1.42–2.44) in that of Kawaguchi et al. [24] (targeting NSCLC). Additionally, two reviews [19, 24] reported that sarcopenic patients were more likely to withstand major complications according to a single study on 328 patients with NSCLC (16.1% vs. 7.1%, p = 0.036) [42]. Lower the SMI/PMI threshold for diagnosing sarcopenia, higher was the risk of enduring postoperative complications in NSCLC [24].
3.6.2 Overall response rate
The overall response rate refers to the percentage of patients whose tumors disappear (complete response) or decrease in size (partial response) after treatment. The disease control rate describes the proportion of patients with decreased or stable disease burden during the study period [45]. The endpoints in patients with NSCLC receiving immunotherapy were condensed in two meta-analyses [21, 27]; they revealed a significantly worse disease control rate in sarcopenic versus non-sarcopenic participants. Pre-treatment sarcopenia and deteriorating sarcopenic status after initiating therapies were linked to a decreased disease control rate (risk ratio [RR]: 0.62, 95% CI: 0.19–1.53) [21]. However, although sarcopenia showed an unfavorable overall response rate (RR: 0.54, 95% CI: 0.19–0.53), the difference between sarcopenic and non-sarcopenic patients was not statistically significant. Interestingly, a pooled result from three studies [38, 46, 47] suggested that sarcopenia did not increase the rate of immune-related adverse events (RR: 0.99, 95% CI: 0.21–4.67) such as dermatitis, colitis, pneumonitis, or endocrinopathies [21].
3.6.3 Progression-free survival
Progression-free survival implies the time before the detection of disease progression or patient’s death [48]. The duration without tumor relapse after treatment is represented by disease-free survival [49]. Pre-treatment sarcopenia was significantly related to shortened progression-free survival rates in patients with lung cancer receiving immunotherapy in the meta-analyses by Wang et al. [21], Deng et al. [23], Lee et al. [25] and Takenaka et al. [27]. The association of sarcopenia with disease-free survival varied among different patient populations. Deng et al. [18] and Yang et al. [20] did not acknowledge a significant difference in the postoperative disease-free survival between sarcopenic and non-sarcopenic patients with NSCLC [18, 20]. However, Kawaguchi et al.[24] reported that patients with NSCLC and sarcopenia had reduced disease-free survival after lung resections (OR: 1.66, 95% CI: 1.00–2.74). Poorer disease-free survival was also observed in sarcopenic patients with advanced NSCLC on immune checkpoint inhibitors (ICIs) with a hazard ratio (HR) of 1.98 (95% CI: 1.32–2.97) [21].
3.6.4 Overall survival
Patients with lung cancer and concomitant sarcopenia had worse overall survival than non-sarcopenic patients as demonstrated repeatedly in our umbrella review. Across various meta-analyses, the pooled HR and RR of mortality for sarcopenic patients ranged between 1.27–4.68 and 1.63–2.15, respectively [17,18,19,20,21,22,23,24,25, 27]. Buentzel et al. [17] studied patients with lung cancer receiving diverse anti-cancer therapies (surgery, targeted therapy, chemotherapy, radiotherapy, or a combination) and discovered that sarcopenia was an independent risk factor for mortality (HR: 3.13, 95% CI: 2.06–4.76). From 11 studies, Yang et al. [20] combined data from 1,621 patients with lung cancer and illustrated a significantly worse overall survival for those with sarcopenia (HR: 2.23, 95% CI: 1.68–2.94) than those without. Further subgroup analysis did not reveal any discrepancy by tumor type (NSCLC vs. SCLC) or staging (stage I–II vs. stage III–IV).
Regarding cancer treatment, sarcopenia was significantly associated with poor overall survival among either operated [18, 19, 24] or immunotherapy-managed [21, 23, 27] patients with NSCLC. Wang et al. [21] showed that sarcopenia was an independent unfavorable prognostic factor for patients with NSCLC on ICIs with an HR of 1.61(95% CI: 1.24–2.10) and it indicated higher mortality for the subgroup using nivolumab (HR: 2.10, 95% CI: 1.22–3.61). Buentzel et al. [17] and Yang et al. [20] reported that the cancer stage did not affect the predictability of sarcopenia for mortality. Deng et al. [18] noted that this was especially true for patients with stage I disease. In their meta-analysis, sarcopenia led to significantly poorer overall survival in patients with stage I NSCLC (RR: 2.09, 95% CI: 1.51–2.88). However, the correlation was not significant when studies recruiting NSCLC patients of all stages were analyzed (RR: 1.37, 95% CI: 0.78–2.42) [18]. For every one unit fall in SMA and SMI or for a one-degree decrease in the phase angle by BIA during the treatment for lung cancer, a 4% increase in mortality was observed [17]. Wang et al. [21] also observed that the presence of muscle loss under immunotherapy was predictive for poor overall survival (HR: 4.97, 95% CI: 2.39–10.32). Nonetheless, there were inconsistent findings regarding the median overall survival [20]. Sarcopenic patients had significantly poorer median overall survival than non-sarcopenic patients in SCLC (8.6 vs. 16.8 months, p = 0.031) [50], stage I NSCLC (32 vs. 112 months, p < 0.01) [51] and stage IV NSCLC (12.6 vs. 23.5 months, p = 0.035) [52] cohorts. However, the difference was not significant in stage IIIB–IV NSCLC (7.5 vs. 7.9 months, p = 0.490) [32] (Table 4).
4 Discussion
According to this umbrella review, sarcopenia was prevalent among patients with lung cancer and served as an unfavorable prognostic factor. Similarly, sarcopenia was significantly associated with higher postoperative complications, lower disease control rates in patients using ICIs, and poorer overall survival. However, it does not increase the risk of immune-related side effects in patients receiving ICIs for lung cancer. The predictive value of sarcopenia for increased mortality remained unchanged across patients with different tumor types or those using distinct anti-cancer therapies. The findings of this umbrella review are summarized in Fig. 2.
We highlighted the pervasiveness of muscle depletion in lung cancer, with an overall prevalence of sarcopenia ranging from 42.8 to 45.0%. More patients with advanced disease were sarcopenic than those with early stage lung cancer. Sarcopenia is a part of the multifactorial cachexia syndrome. Patients suffering from cachexia experience profound body weight loss, primarily from the wasting of skeletal muscle and adipose tissue, anemia, and extra-cellular fluid imbalance [53]. The prevalence of cachexia ranges from 36 to 61% in NSCLC [54,55,56]. Anorexia, accelerated resting energy expenditure, increased lipolysis, and depression of protein synthesis coupled with rising protein degradation play a role in the development of cachexia [57]. Herein, although cachexic patients are known to be sarcopenic, the majority of sarcopenic people may not be cachexic [58]. Changes in the intertwined epigenic, cellular, and hormonal pathways of skeletal muscle metabolism that induce sarcopenia are not yet fully understood [11]. Immobility and insufficient calorie intake are the primary driving causes [59]. In patients with lung cancer, malignancy related pain, fatigue, and depression could lead to disuse atrophy. The side effects of antineoplastic therapy, such as nausea, vomiting, and altered taste exacerbate malnutrition. Reduced muscle strength hinders ambulatory ability, creating a disabling vicious cycle.
In our umbrella review, we noticed that various criteria had been employed to define sarcopenia in patients with lung cancer. The muscle mass at the third lumbar vertebra level (L3) upon CT imaging was the mostly used standard because it closely reflected the whole body fat-free mass [60]. Instead of the L3 landmark, some researchers calculated the mass at the thoracic muscle because it is related to the respiratory muscle condition [19]. Nishumura et al. [19] emphasized that the vertebral level of measurement did not interfere with the predictive value of sarcopenia for postoperative complications.
A handful of techniques can be used to determine body composition. Although DEXA and BIA are cost-effective, their estimations can be altered by the individual’s hydration status (which is often abnormal in the ill) along with the inconsistencies across different instrument brands and reference populations [11]. In contrast, CT can provide detailed imaging of specific tissues. Moreover, the examination is routinely performed throughout the cancer workup and follow-up. However, CT only measures the muscle quantity. It is unclear whether the diagnosis of sarcopenia, without assessing muscle strength, affects the predictive value. Additionally, there was considerable heterogeneity in the cutoff values, among which the L3 SMI thresholds proposed by Prado et al. [30] (men: < 52.4 cm2/m2; women < 38.5 cm2/m2) and Martin et al. [31] (men: SMI < 43 cm2/m2 for those with body mass index [BMI] < 25 kg/m2, SMI < 53 cm2/m2 for those with BMI ≥ 25 kg/m2; women: SMI < 41 cm2/m2) were the most widely adopted. Kawaguchi et al. [24] suggested that L3 PMIs < 6.36 cm2/m2 for men, < 3.92 cm2/m2 for women and < 3.70 cm2/m2 for men, < 2.50 cm2/m2 for women were optimal for predicting survival and postoperative complications, respectively. Further studies are needed to establish the most suitable cutoff values of lean body mass for the association of various prognostic parameters in patients with lung cancer.
4.1 The impact of sarcopenia on the prognosis of lung cancer
Sarcopenia is a strong predictor of increased postoperative complications. Prior studies have delineated the deteriorating influence of sarcopenia on invasive procedures, such as hip fracture surgery, emergent abdominal surgery, and gastrectomy for cancer [61,62,63]. Adequate nutrition and tissue perfusion are the basis for wound healing. However, sarcopenia is associated with anemia; therefore, it impedes tissue regeneration [64]. Respiratory muscles of sarcopenic patients are weakened by hypercatabolic state and increased levels of pro-inflammatory cytokines such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and transforming growth factor (TGF)-β [65]. The ensuing difficulty of weaning from ventilator support could predispose patients to further deconditioning, pulmonary infections, longer intensive care unit stay, and ultimately death. The risk of acute respiratory failure and 30-day mortality were significantly higher in sarcopenic patients with lung cancer after pneumonectomy [39].
Current evidence suggests that patients with NSCLC and sarcopenia have inferior responsiveness to immunotherapy and progression-free survival. The goal of immunotherapy is to enhance immune surveillance, such as deploying T cells to eradicate cancer cells [66]. Muscles regulate the immune response by signaling soluble myokines, cell surface molecules, and cell-to-cell interactions [67]. Wasting of skeletal muscles is likely to disrupt the equilibrium of muscle-immune systems and impair immune cell production. Furthermore, T cells become functionally incompetent in patients with cancers due to this miscommunication between skeletal muscles and lymphoid organs [68]. The “exhausted” T cells may in turn compromise the efficacy of immunotherapy [68]. The action of immunotherapy in patients with lung cancers may also be modulated by the gut and lung microbiome (gut-lung axis) [69]. Malnutrition, chronic infections, and antibiotic overuse presumably distort the intrinsic gut ecosystem, leading to a subsequent pro-inflammatory status and sarcopenia [69].
There are inconsistent results regarding the association between sarcopenia and disease-free survival in patients with lung cancer. This may be due to the limited number of original studies conducted in the early years. In the meta-analyses by Deng et al. [18] and Yang et al. [20], disease-free survival was computed from the same three studies [33, 50, 51]. Although both reviews noted a trend towards poor disease-free survival for sarcopenic patients, neither of them revealed a statistically significant difference. Later, Kawaguchi et al. [24] demonstrated shortened disease-free survival for sarcopenic patients with surgically treated NSCLC based on six studies [33, 43, 50, 51, 70, 71]. Nevertheless, our umbrella review showed that meta-analyses on the direct impact of sarcopenia on cancer recurrence, distant metastasis, and toxicity of chemotherapy and radiotherapy were lacking.
Sarcopenia predicted poor overall survival in patients with lung cancer; similarly, sarcopenia had a negative impact on the survival of patients with operated NSCLC. Although Deng et al. [18] reported that the predictive value was more robust for stage I patients, merely one study [72] analyzing stage I–IV patients reported no significant impact of sarcopenia on the overall survival. The prognosis was also inferior in sarcopenic patients with NSCLC receiving immunotherapy. Notably, there are limited data on the survival outcomes of patients receiving chemotherapy. The mechanism by which loss of muscle mass shortens lung cancer survival can be interpreted in several ways. First, sarcopenia on its own is related to increased all-cause mortality regardless of age and sex [73]. Second, performance status, which has recently been included in the recent diagnostic criteria of the Asian Working Group for Sarcopenia, is recognized as a prognostic factor for lung cancer [74]. Deteriorated physiological reserve, a hallmark of frailty and sarcopenia, lowers the patient’s tolerance to aggressive therapeutic approaches, resulting in substandard dosing or premature treatment termination. Studies have shown that sarcopenic cancer patients had poor compliance during chemotherapy [75]. Lastly, hampered treatment response and added complication risks in sarcopenic patients, as also shown in our review, have adverse effects on cancer prognosis.
Our umbrella review has some limitations. First, it was inherently subject to biases in the included systematic reviews and meta-analyses. Complex interactions among skeletal muscles, inflammation, and the immune system are elusive; thus, there is a knowledge gap between the mechanism and treatment of sarcopenia in patients with lung cancer. Further research is needed to clarify the influence of sarcopenia on metastasis, recurrence, treatment response/toxicity, and quality of life in patients with lung cancer. Likewise, future studies verifying the predictive power of sarcopenia for various clinical outcomes in different subtypes and stages of lung cancer are also needed.
5 Conclusions
Sarcopenia is a major health threat in lung cancer, affecting up to half of all patients. Its diagnosis in this population should not be underestimated because of its association with elevated postoperative complications, decreased immunotherapy response rates, and increased mortality. In patients with sarcopenia and lung cancer, survival is adversely affected regardless of the cancer type (NSCLC/SCLC), stage, or treatment option. Therefore, sarcopenia is a robust prognostic factor for therapeutic responses and outcomes in patients with lung cancer. Further research is needed regarding the pathophysiology and interventions in the lung cancer population.
Data availability
Not applicable.
Code availability
Not applicable.
References
Bade BC, Dela Cruz CS. Lung cancer 2020: epidemiology, etiology, and prevention. Clin Chest Med. 2020;41(1):1–24.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol (Pozn). 2021;25(1):45–52.
Time CS. SEER cancer statistics review 1975–2008. 2011.
Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. Lung Cancer. 2016:47–75.
Simmons CP, Koinis F, Fallon MT, Fearon KC, Bowden J, Solheim TS, et al. Prognosis in advanced lung cancer–a prospective study examining key clinicopathological factors. Lung Cancer. 2015;88(3):304–9.
Shepshelovich D, Xu W, Lu L, Fares A, Yang P, Christiani D, et al. Body Mass Index (BMI), BMI change, and overall survival in patients with SCLC and NSCLC: a pooled analysis of the International Lung Cancer Consortium. J Thorac Oncol. 2019;14(9):1594–607.
Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90(10):1905–11.
Kara M, Kaymak B, Frontera W, Ata AM, Ricci V, Ekiz T, et al. Diagnosing sarcopenia: functional perspectives and a new algorithm from the ISarcoPRM. J Rehabil Med. 2021;53(6):jrm00209.
Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):177.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
Han DS, Wu WT, Hsu PC, Chang HC, Huang KC, Chang KV. Sarcopenia is associated with increased risks of rotator cuff tendon diseases among community-dwelling elders: a cross-sectional quantitative ultrasound study. Front Med (Lausanne). 2021;8: 630009.
Beaudart C, Zaaria M, Pasleau F, Reginster J-Y, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS ONE. 2017;12(1): e0169548.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):1–11.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358: j4008.
Collins J, Noble S, Chester J, Coles B, Byrne A. The assessment and impact of sarcopenia in lung cancer: a systematic literature review. BMJ Open. 2014;4(1): e003697.
Buentzel J, Heinz J, Bleckmann A, Bauer C, Röver C, Bohnenberger H, et al. Sarcopenia as prognostic factor in lung cancer patients: a systematic review and meta-analysis. Anticancer Res. 2019;39(9):4603–12.
Deng HY, Hou L, Zha P, Huang KL, Peng L. Sarcopenia is an independent unfavorable prognostic factor of non-small cell lung cancer after surgical resection: a comprehensive systematic review and meta-analysis. Eur J Surg Oncol. 2019;45(5):728–35.
Nishimura JM, Ansari AZ, D’Souza DM, Moffatt-Bruce SD, Merritt RE, Kneuertz PJ. Computed tomography-assessed skeletal muscle mass as a predictor of outcomes in lung cancer surgery. Ann Thorac Surg. 2019;108(5):1555–64.
Yang M, Shen Y, Tan L, Li W. Prognostic value of sarcopenia in lung cancer: a systematic review and meta-analysis. Chest. 2019;156(1):101–11.
Wang J, Cao L, Xu S. Sarcopenia affects clinical efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients: a systematic review and meta-analysis. Int Immunopharmacol. 2020;88: 106907.
Au PC-M, Li H-L, Lee GK-Y, Li GH-Y, Chan M, Cheung BM-Y, et al. Sarcopenia and mortality in cancer: a meta-analysis. Osteoporos sarcopenia. 2021;7:S28–33.
Deng HY, Chen ZJ, Qiu XM, Zhu DX, Tang XJ, Zhou Q. Sarcopenia and prognosis of advanced cancer patients receiving immune checkpoint inhibitors: a comprehensive systematic review and meta-analysis. Nutrition. 2021;90: 111345.
Kawaguchi Y, Hanaoka J, Ohshio Y, Okamoto K, Kaku R, Hayashi K, et al. Does sarcopenia affect postoperative short- and long-term outcomes in patients with lung cancer? A systematic review and meta-analysis. J Thorac Dis. 2021;13(3):1358–69.
Lee D, Kim NW, Kim JY, Lee JH, Noh JH, Lee H, et al. Sarcopenia’s prognostic impact on patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J Clin Med. 2021;10(22):5329.
McGovern J, Dolan RD, Horgan PG, Laird BJ, McMillan DC. Computed tomography-defined low skeletal muscle index and density in cancer patients: observations from a systematic review. J Cachexia Sarcopenia Muscle. 2021;12(6):1408–17.
Takenaka Y, Oya R, Takemoto N, Inohara H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle. 2021;12(5):1122–35.
Surov A, Wienke A. Prevalence of sarcopenia in patients with solid tumors: A meta-analysis based on 81,814 patients. JPEN J Parenter Enteral Nutr. 2022. https://doi.org/10.1002/jpen.2415.
Voorn MJJ, Beukers K, Trepels CMM, Bootsma GP, Bongers BC, Janssen-Heijnen MLG. Associations between pretreatment nutritional assessments and treatment complications in patients with stage I–III non-small cell lung cancer: A systematic review. Clin Nutr ESPEN. 2022;47:152–62.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35.
Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–47.
Stene GB, Helbostad JL, Amundsen T, Sørhaug S, Hjelde H, Kaasa S, et al. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol. 2015;54(3):340–8.
Suzuki Y, Okamoto T, Fujishita T, Katsura M, Akamine T, Takamori S, et al. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer. 2016;101:92–7.
Kim EY, Kim YS, Seo J-Y, Park I, Ahn HK, Jeong YM, et al. The relationship between sarcopenia and systemic inflammatory response for cancer cachexia in small cell lung cancer. PLoS ONE. 2016;11(8): e0161125.
Sjøblom B, Grønberg BH, Wentzel-Larsen T, Baracos VE, Hjermstad MJ, Aass N, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr. 2016;35(6):1386–93.
Srdic D, Plestina S, Sverko-Peternac A, Nikolac N, Simundic A-M, Samarzija M. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer—chemotherapy toxicity and prognostic value. Support Care Cancer. 2016;24(11):4495–502.
Cortellini A, Palumbo P, Porzio G, Verna L, Giordano AV, Masciocchi C, et al. Single-institution study of correlations between skeletal muscle mass, its density, and clinical outcomes in non-small cell lung cancer patients treated with first-line chemotherapy. Thoracic Cancer. 2018;9(12):1623–30.
Cortellini A, Verna L, Porzio G, Bozzetti F, Palumbo P, Masciocchi C, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: a “hypothesis-generator” preliminary report. Thoracic Cancer. 2019;10(2):347–51.
Martini K, Chassagnon G, Fournel L, Prieto M, Hoang-Thi T-N, Halm N, et al. Sarcopenia as independent risk factor of postpneumonectomy respiratory failure. ARDS Mortal Lung Cancer. 2020;149:130–6.
Takada K, Yoneshima Y, Tanaka K, Okamoto I, Shimokawa M, Wakasu S, et al. Clinical impact of skeletal muscle area in patients with non-small cell lung cancer treated with anti-PD-1 inhibitors. J Cancer Res Clin Oncol. 2020;146(5):1217–25.
Kim E, Lee H, Kim K, Lee J-I, Kim Y, Choi W-J, et al. Preoperative computed tomography-determined sarcopenia and postoperative outcome after surgery for non-small cell lung cancer. Scand J Surg. 2018;107(3):244–51.
Nakamura R, Inage Y, Tobita R, Yoneyama S, Numata T, Ota K, et al. Sarcopenia in resected NSCLC: effect on postoperative outcomes. J Thorac Oncol. 2018;13(7):895–903.
Shinohara S, Otsuki R, Kobayashi K, Sugaya M, Matsuo M, Nakagawa M. Impact of sarcopenia on surgical outcomes in non-small cell lung cancer. Ann Surg Oncol. 2020;27(7):2427–35.
Icard P, Schussler O, Loi M, Bobbio A, Mansuet Lupo A, Wislez M, et al. Pre-disease and pre-surgery BMI, weight loss and sarcopenia impact survival of resected lung cancer independently of tumor stage. Cancers. 2020;12(2):266.
Sznol M. Reporting disease control rates or clinical benefit rates in early clinical trials of anticancer agents: useful endpoint or hype? Curr Opin Investig Drugs. 2010;11(12):1340–1.
Tsukagoshi M, Yokobori T, Yajima T, Maeno T, Shimizu K, Mogi A, et al. Skeletal muscle mass predicts the outcome of nivolumab treatment for non-small cell lung cancer. Medicine. 2020;99(7):e19059.
Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67.
Fleming TR, Rothmann MD, Lu HL. Issues in using progression-free survival when evaluating oncology products. J Clin Oncol. 2009;27(17):2874–80.
Cerfolio RJ, Bryant AS. Predictors of survival and disease-free survival in patients with resected N1 non-small cell lung cancer. Ann Thorac Surg. 2007;84(1):182–90.
Kim EY, Kim YS, Park I, Ahn HK, Cho EK, Jeong YM. Prognostic Significance of CT-determined sarcopenia in patients with small-cell lung cancer. J Thorac Oncol. 2015;10(12):1795–9.
Tsukioka T, Nishiyama N, Izumi N, Mizuguchi S, Komatsu H, Okada S, et al. Sarcopenia is a novel poor prognostic factor in male patients with pathological Stage I non-small cell lung cancer. Jpn J Clin Oncol. 2017;47(4):363–8.
Rossi S, Di Noia V, Tonetti L, Strippoli A, Basso M, Schinzari G, et al. Does sarcopenia affect outcome in patients with non-small-cell lung cancer harboring EGFR mutations? Future Oncol. 2018;14(10):919–26.
Tisdale MJ. Cancer anorexia and cachexia. Nutrition. 2001;17(5):438–42.
von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1(1):1–5.
Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am J Med. 1980;69(4):491–7.
Granda-Cameron C, DeMille D, Lynch MP, Huntzinger C, Alcorn T, Levicoff J, et al. An interdisciplinary approach to manage cancer cachexia. Clin J Oncol Nurs. 2010;14(1):72–80.
Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89(2):381–410.
Muscaritoli M, Anker S, Argiles J, Aversa Z, Bauer J, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG)“cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr. 2010;29(2):154–9.
Cederholm T, Morley JE. Sarcopenia: the new definitions. Curr Opin Clin Nutr Metab Care. 2015;18(1):1–4.
Mourtzakis M, Prado CMMPMM, Lieffers JRLR, Reiman T, McCargar LJMJ, Baracos VEBE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33(5):997–1006.
Kim YK, Yi SR, Lee YH, Kwon J, Jang SI, Park SH. Effect of sarcopenia on postoperative mortality in osteoporotic hip fracture patients. J Bone Metab. 2018;25(4):227–33.
Rangel EL, Rios-Diaz AJ, Uyeda JW, Castillo-Angeles M, Cooper Z, Olufajo OA, et al. Sarcopenia increases risk of long-term mortality in elderly patients undergoing emergency abdominal surgery. J Trauma Acute Care Surg. 2017;83(6):1179–86.
Shen Y, Hao Q, Zhou J, Dong B. The impact of frailty and sarcopenia on postoperative outcomes in older patients undergoing gastrectomy surgery: a systematic review and meta-analysis. BMC Geriatr. 2017;17(1):188.
Moon J-H, Kong M-H, Kim H-J. Relationship between low muscle mass and anemia in Korean elderly men: using the Korea National Health and Nutrition Examination Survey (KNHANES IV–V). J Clin Gerontol Geriatrics. 2015;6(4):115–9.
Barnes P, Celli B. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–85.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68.
Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–8.
Wu J, Weisshaar N, Hotz-Wagenblatt A, Madi A, Ma S, Mieg A, et al. Skeletal muscle antagonizes antiviral CD8+ T cell exhaustion. Sci Adv. 2020;6(24):3458.
Nigro E, Perrotta F, Scialò F, D’Agnano V, Mallardo M, Bianco A, et al. Food, nutrition, physical activity and microbiota: which impact on lung cancer? Int J Environ Res Public Health. 2021;18(5):2399.
Ozeki N, Kawaguchi K, Fukui T, Nakamura S, Hakiri S, Mori S, et al. Psoas muscle mass in patients undergoing lung cancer surgery: a prognostic difference between squamous cell carcinoma and adenocarcinoma. Int J Clin Oncol. 2020;25(5):876–84.
Tsukioka T, Izumi N, Mizuguchi S, Kyukwang C, Komatsu H, Toda M, et al. Positive correlation between sarcopenia and elevation of neutrophil/lymphocyte ratio in pathological stage IIIA (N2-positive) non-small cell lung cancer patients. Gen Thorac Cardiovasc Surg. 2018;66(12):716–22.
Kim EY, Lee HY, Kim KW, Lee JI, Kim YS, Choi WJ, et al. Preoperative computed tomography-determined sarcopenia and postoperative outcome after surgery for non-small cell lung cancer. Scand J Surg. 2017;107(3):244–51.
Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42(2):203–9.
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300-7.e2.
Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. 2017;28(9):2107–18.
Funding
This work was supported by National Taiwan University Hospital, Bei-Hu Branch; Ministry of Science and Technology (Grant Number: MOST 106-2314-B-002-180-MY3 and 109-2314-B-002-114-MY3); and the Taiwan Society of Ultrasound in Medicine.
Author information
Authors and Affiliations
Contributions
T-YL: Data curation; Investigation; Writing—original draft. Y-FC: Writing—review & editing. W-TW: Data curation; Investigation. D-SH: Conceptualization. I-CT: Visualization. K-VC: Funding acquisition; Methodology; Writing—review & editing. LÖ: Data curation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Keywords and search results in different databases. Table S2. Excluded reviews and reasons. Table S3. Original studies in the included reviews.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, TY., Chen, YF., Wu, WT. et al. Impact of sarcopenia on the prognosis and treatment of lung cancer: an umbrella review. Discov Onc 13, 115 (2022). https://doi.org/10.1007/s12672-022-00576-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12672-022-00576-0