Abstract
Colorectal carcinoma is the second leading cause of cancer-related deaths, and indeed, rectal cancer accounting for approximately one third of newly diagnosed patients. Gold standard in the treatment of rectal cancer is a multimodality approach, aiming at a good control of the local disease. Distant recurrences are the major cause of mortality. Currently, Locally Advanced Rectal Cancer (LARC) patients undergo a combined treatment of chemotherapy and radiotherapy, followed by surgery. Eventually, more chemotherapy, namely adjuvant chemotherapy (aCT), may be necessary. Total Neoadjuvant Therapy (TNT) is an emerging approach aimed to reduce distant metastases and improve local control. Several ongoing studies are analyzing whether this new approach could improve oncological outcomes. Published results were encouraging, but the heterogeneity of protocols in use, makes the comparison and interpretation of data rather complex. One of the major concerns regarding TNT administration is related to its effect on larger and more advanced cancers that might not undergo similar down-staging as smaller, early-stage tumors. This minireview, based on a systematic literature search of randomized clinical trials and meta-analysis, summarizes current knowledge on TNT. The aim was to confirm or refute whether or not current practice of TNT is based on relevant evidence, to establish the quality of that evidence, and to address any uncertainty or variation in practice that may be occurring. A tentative grouping of general study characteristics, clinical features and treatments characteristics has been undertaken to evaluate if the reported studies are sufficiently homogeneous in terms of subjects involved, interventions, and outcomes to provide a meaningful idea of which patients are more likely to gain from this treatment.
Similar content being viewed by others
Introduction
Core tip: Given the enormous amount of scientific information published every year, systematic reviews and meta-analyses have become indispensable methods for the evaluation of medical treatments and the delivery of the best evidence-based practice. Total Neoadjuvant Therapy (TNT) is an emerging approach for the treatment of Locally Advanced Rectal Cancer (LARC) aimed at improving oncological results. One of the major concerns regarding its administration is related to its effect on larger and more advanced cancers that might not undergo similar down-staging effect as smaller, early-stage tumors. For this reason, using the available evidence, we propose an interesting minireview which is also novel, ethical and relevant on this particularly complex intervention with the scope of summarizing the body of research to evaluate sources of heterogeneity.
Colorectal cancer is currently the third most prevalent cancer worldwide, with a particularly high incidence in western countries, possibly due to the concurrent increase of obesity and metabolic syndrome [1,2,3,4,5,6]. In Europe, the gold standard for the treatment of locally advanced rectal cancer (LARC) consists in a multidisciplinary approach based on the administration of either preoperative long-course chemoradiotherapy (CRT) or short-course radiotherapy (SCRT), followed by surgery and adjuvant chemotherapy [7,8,9,10].
Improved therapeutic strategies and multimodality approach have led to a better local control of the disease, thus reducing the 5 years local recurrence rate from 27 to 3.7% [11]. Preoperative radiotherapy contributed to a better control of local disease [12], but choice of the best protocol of administration and timing of surgery is still a matter of debate [7, 13,14,15,16]. Surgery has also played a major role since the introduction of total mesorectal excision [8] and, laparoscopic and robotic procedures can now be performed achieving the same oncological results in referral centers [17].
Despite several efforts and the use of a multimodality approach, distant recurrences are still significant and represent the leading cause of mortality for rectal cancer patients [18,19,20]. In this scenario, chemotherapy (CT) plays an important role since it could allow a better control of systemic disease, improving overall survival (OS), disease free survival (DFS) and distant recurrence rate. To date, most national guidelines include both neoadjuvant and adjuvant CT for LARC [7, 8, 21], despite lack of evidence on the true benefits of adjuvant CT (aCT) in patients who have already received neoadjuvant chemotherapy (nCT) [22,23,24,25,26]. However, three quarter of the patients will eventually receive aCT after surgery but less than half will complete the planned treatment [22].
Several randomized clinical trials (RCTs) aimed at optimizing LARC treatment are focusing on the intensification of neoadjuvant treatment with standard dose polychemotherapy administration before surgery, which is known as total neoadjuvant treatment (TNT). Several reasons support TNT administration:
-
Potential early treatment of occult micro-metastases to improve systemic disease control
-
Increase patient tolerance and compliance to CT because administered preoperatively
-
Ease surgery, reducing tumor bulk and nodes
-
Increase sphincter sparing procedures rate and increase organ preservation rate for patients with complete clinical response (cCR).
However, despite encouraging results, not all patients respond equally to TNT and results are variable. Existence of a spectrum of local response to TNT is well known, ranging from complete pathological response (pCR) and near-complete pathological response (npCR) to non-response. One of the major concerns regarding the administration of TNT is related to its effect on larger and more advanced rectal cancers that seem to have a worse response than smaller, early-stage tumors.
Furthermore, TNT could impact on patient performance status, thus reducing the number of patients able to tolerate surgery. In addition, non-responders can both experience micro metastases growth and local tumor progression, jeopardizing surgical treatment for those patients with previously resectable tumors. One last consideration is on potentially unnecessary overtreatments if we consider those patients that have already done well after the initial neoadjuvant treatment [27,28,29].
It seems therefore, important to identify factors predictive of a good response to TNT. Accurate analysis of clinical studies and treatments characteristics is necessary to evaluate interventions and outcome in order to offer a meaningful idea of which patients are more likely to gain from this treatment.
Methods
Literature search
A systematic literature search was carried out on Pubmed for articles published up to December 31, 2021. The following Medical Subject Headings (MeSH) terms were used: “total neoadjuvant therapy” OR “total neoadjuvant treatment” OR “neoadjuvant” AND “rectal cancer” OR “locally advanced rectal cancer”. A further search was performed in clinicaltrial.gov using the terms “total neoadjuvant therapy” and “rectal cancer”. References of the included studies were manually assessed in order to detect any missing studies.
Inclusion and exclusion criteria
Only RCTs, systematic reviews and meta-analysis in the English language were included in the literature search. In case of duplications only the most recent or most detailed study was included. Furthermore, all selected articles had to meet all the following inclusion criteria: (1) LARC, defined as Stage II/III; (2); RCTs comparing standard CRT in the control arm versus TNT in the experimental arm.
Exclusion criteria were the following: (1) use of any additional biological drugs both in the experimental arm or in the control arm; (2) RCTs including organ preservation alone after neoadjuvant treatment.
Data extraction
Extracted variables were the following: general study characteristics (e.g., author, name of the study, country of recruitment, year of last publication, study design, number of patients, treatment arms and primary end point), treatment protocols (RT regimens, CT agents, timing of CT administration, timing of surgery, aCT), local disease control outcomes (pCR, nodal down staging, resection, lymphovascular and perinervous invasion, local recurrence rate), distant disease control outcomes (DFS, OS, distant recurrence rate), toxicity and complications (chemo-related adverse effect, surgical complications, compliance), and predictors of disease control.
Results of literature search
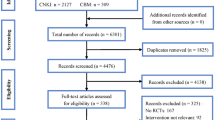
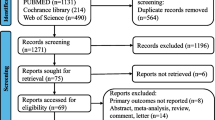
Three hundred and sixty-four articles from PubMed and one hundred and thirty-four from clinicaltrial.gov were analyzed for language and article relevance depending on both title and abstract. The publication year ranged from 2011 to 2021. Five meta-analysis and eight RCTs met the inclusion’s criteria. The full detail of the inclusions process and PRISMA flow chart can be found in Fig. 1. All RCTs were included for descriptive analysis. The number of recruited patients in each study ranged from 49 to 912; totally, data regarding 2705 subjects was collected.
Collected data show wild heterogeneity in terms of RT and CT regimens, CT agents, timing of CT administration and timing of surgery for both experimental TNT and standard therapy.
Treatment protocols for larc
Treatment protocols show high heterogeneity. In particular, protocols differ in many respects including type of radiotherapy (SCRT vs long RT plus CT), use of chemotherapeutic drugs, timing of CT administration (induction vs consolidation), timing of surgery and use of adjuvant therapy. Furthermore, differences can be found within the same study, due to the different chemo-radio therapeutic regimens employed for the two arms.
General features of the included studies are showed in Tables 1 and 2.
Radiotherapy regimens
Two different RT regimens (SCRT and CRT) are considered the standard of care for the treatment of LARC [7].
Most of the analyzed studies provided CRT both in the standard and in the experimental arm. Only two randomized phase III studies provided SCRT in the experimental arm, before the administration of consolidation CT [13, 30]. Moreover, the Chinese study by Deng et al., that used CRT for the two arms of the study, was the only one including a further experimental arm without neoadjuvant RT administration [31].
Outcomes of SCRT vs CRT are controversial. According to Kong et al. studies using SCRT reported an 86% increase in local recurrence rates compared to CRT (9.3% vs 5.3%) [27]. In contrast, Liu et al. found SCRT to lead to higher pCR rates compared to CRT [32].
CRT administration is strongly recommended if CRM and R0 resection are predicted at risk independently from T and N stage [7].
CT agents
Continuous intravenous infusions of 5-fluorouracil (5FU) or oral capecitabine during CRT are strongly recommended [7]; on the contrary, statements regarding the administration of other CT agents are lacking or are based on low-grade recommendations (Tables 3, 4, 5).
CRT was based on single agent administration (5FU or capecitabine) in 6 out of 8 studies [30, 31, 33,34,35,36]. In all these studies CRT agents were the same for both the experimental and standard arm, except for the RAPIDO trial, which compared capecitabine-based CRT in the control arm to SCRT in the experimental arm [30]. Two RCTs used multiagent oxaliplatin-based CRT: the GCR-3 study administered capecitabine and oxaliplatin (CAPOX/XELOX) both in the experimental and standard arm [37], while the POLISH II compared 5FU/folinic acid and oxaliplatin (FOLFOX) in the control arm to SCRT in the experimental group [13].
In almost all the analyzed studies TNT consisted in the administration of multi-agent oxaliplatin-based chemotherapeutic agents. Only Moore et al. chose 5FU administration for both CRT and TNT treatment [33]. The others analyzed both CAPOX/XELOX or FOLFOX administration. The administration of 5FU/folinic acid, irinotecan and oxaliplatin (FOLFIRINOX) was used only in the PRODIGE trial [36].
The number of administered cycles and thus length of TNT widely differed too, ranging from 6 weeks for the administration of 3 cycles of 5FU in the WAIT trial [33], to 18 weeks for the administration of 6 cycles of CAPOX or 9 cycles of FOLFOX in the RAPIDO trial [30].
A recent meta-analysis of RCTs stratified pCR results depending on TNT length in terms of weeks and found that patients receiving less than 12 weeks of therapy showed no significant differences in terms of pCR rates compared to standard treatment [32].
The vast inter-study variation in CT regimens contributes to difficult interpretation of chemotherapeutic agents’ effect on primary and secondary outcomes.
Timing of TNT administration
One of the most discussed clinical points is whether to administer TNT before or after neoadjuvant RT. In fact, induction-type TNT, which is given before neoadjuvant RT, allows an early systemic disease control, slowing occult micro metastasis growth, thus potentially reducing distant failure rates, but could determine local growth of previously resectable cancers. On the contrary, consolidation-type TNT, which is administered during the free interval between the end of neoadjuvant RT and surgery, could increase the chemoradiation-to-surgery interval but could also improve pCR after radiation and therefore increase sphincter-preserving rate.
Of the 8 RCT, four reported on induction and four on consolidation TNT.
The recent meta-analysis by Kong et al. comparing TNT to the standard of care for the treatment of LARC stratified results of both short and long-term outcomes depending on TNT type and found that induction regimens only slightly improve pCR (28%) and increase negative CRM rates but have no impact on long-term outcomes. On the other hand, patients undergoing consolidation-type TNT showed significant reduction in the odds of distant recurrence (27%), 90% increase in pCR at the cost of 86% higher likelihood of local recurrence [27]. Local recurrence rates though were available only for studies using SCRT in their consolidation regimen and this might also have influenced outcomes [13, 30]. Keeping in mind the importance of distant recurrence on patient prognosis [22], consolidation-type TNT regimens appear to be the preferable therapeutic option. Data supporting consolidation TNT are reported in two RCTs testing the optimal timing of TNT. Short-term results of CAO/ARO/AIO-12 and OPRA trials showed improved pCR and cCR, improved compliance to CRT and grade 3 to 4 toxicity rate reduction, when comparing consolidation and induction TNT [38, 39]. Recently, the CAO/ARO/AIO-12 trial long-term results confirmed consolidation TNT safety in terms of oncological endpoints, chronic toxicity, quality of life and stool incontinency [40]. However, long-term results in terms of 3-years disease free survival (DFS) from the phase 2 randomized OPRA trial are still awaited.
Timing of surgery
The most advantageous timing of surgery following RT is not clear. Late surgery could be more technically demanding and results in worse TME quality and higher complications due to increased local fibrosis [41, 42]. Furthermore, non-responders could be at risk of both local and distant progression while waiting for surgery. On the contrary, longer free intervals increase effects of RT, augmenting chances to achieve pCR [43].
The TIMING trial, a phase II non-randomized trial, revealed that delivering consolidation TNT and delaying TME to up to 20 weeks after completion of TNT, increases pelvic fibrosis but does not increase the surgical technical difficulty nor the risk of surgical complications. It is not clear whether consolidation TNT or lengthening the chemoradiation-to-surgery interval led to pCR improvement [44].
In the most recent literature, time of surgery varied in the analyzed RCTs from 6 to 12 weeks, except for the RAPIDO trial in which surgery was performed 22 ± 2 weeks after the end of SCRT. The good results of the RAPIDO trial, in particular for what concerned the resection margins, suggest that longer intervals, whilst beneficial in terms of pCR, will not jeopardize surgical outcomes [30]. However, it is not clear whether pCR improvement is due to the administration of TNT or to the prolonged interval. Thus, these data will need validation before being fully applicable to patients at high risk of cancer progression.
aCT
Several evidences suggest a lack of benefits of aCT in patients who have already received nCT [22,23,24,25,26]. Nevertheless, aCT was administered in 4 studies. Two of them provided aCT after surgery only in patients in the control arm [30, 37] but in the RAPIDO trial the choice of aCT based on CAPOX or FOLFOX and whether to use it or not was based on hospital and physician’s preference,. The other 2 studies administered aCT both in the control and experimental arms [31, 36]. Deng et al. administered 7 cycles of 5FU after surgery both in the control arm and in one of the experimental arms, while changing it to 6–8 cycles of mFOLFOX for patients who had not received neoadjuvant RT. Conroy et al. used adjuvant FOLFOX in both groups but patients in the experimental arm were delivered halved doses (6 vs 12 cycles) [36]. Given the high heterogeneity of protocols in use and the inter-variability amongst protocols, it is very difficult to draw sensible conclusions.
Local disease control (LDC)
LDC can be pictured as a composite of different endpoints. More specifically, the main short-term endpoints for LDC, are pCR rates, nodal downstaging, percentage of R0 resection rate and percentage of lymphovascular and perineural invasion. The main long-term endpoint is local recurrence.
pCR
Significance of pCR is well established due to its correlation with long-term oncological outcomes. Seven RCTs reported on pCR, including over 3000 patients. In particular Sauer et al. demonstrated improved 5-years DFS and OS in patients reaching pCR compared to incomplete pCR/non-responders (86% and 88% vs 63% and 76%, respectively) [45].
One meta-analysis, including 28 studies (of which 3 RCTs) confirmed a 39% increment in the odds of pCR (p = 0.01) [46]. The meta-analysis by Liu et al., which included 8 RCTs, showed an overall improved pCR. Sub-meta-analysis of available data underlined better results in terms of pCR when consolidation TNT is administered [32]. Of note, pCR improvement is particularly evident in the three most recent trials, perhaps due to progress in TNT regimens [30, 31, 36].
Nodal down staging
Pathological N-stage after TNT (ypN) was collected by 6 out of 8 studies including 1866 patients, 931 in the experimental arm and 935 in the standard group. Patients achieving ypN0 in the experimental group were 685 vs 630 in the control (74% vs 67%). Overall, TNT does not seem to induce significant nodal down staging. However, sub-analysis of induction TNT had demonstrated that this strategy decreases likelihood of residual nodal disease [27].
Resection limits
Resection limits were available for 2268 patients reported by 6 RCTs showing comparable R0 resections rates. R0 was achieved in 1102 out of 1225 patients (90%) in the experimental arm and 959 out of 1043 patients (92%) in the control arm. Two meta-analysis confirmed no statistically significant differences in TNT and standard arm for what concern the rate of negative resection margins [32, 46].
Invasion (lymphatic, vascular and neural)
Lymphovascular and perineural invasion (PNI) are extremely important to tailor patient treatment due to the increased risk of both local recurrence and metastatic disease.
Unfortunately, they are reported only by the PRODIGE-23 study [36], which showed non-significant differences.
Local recurrence rate
Local recurrence rate is the main long-term endpoint. TNT overall seems to offer improved local control; however local recurrence rates in the long term, are reported only by two RCTs. Results of the GCR-3 do not show any difference in the experimental arm compared to the standard treatment [37]. Unexpectedly, data from the RAPIDO trial shows an increased local recurrence rate for patients undergoing TNT despite the increase in pCR [30]. However, around 60% of patients had threatened/involved mesorectal fascia at diagnosis, but whether these patients accounted for most local failures remains uncertain [30].
Given the little data available on long-term DFS, it is impossible to conclude whether TNT could improve LDC.
As a word of caution, the meta-analysis by Kong et al. put an alert on consolidation regimens because they may threaten local control [27] however, this was not confirmed by the short-term results of the OPRA trial [39].
Distant disease control
Distant recurrence rates still represent the leading cause of mortality for rectal cancer patients [18], thus affecting survival outcomes. The underlying molecular mechanisms are highly complex and seem to involve, for example, redox regulations [47,48,49], p53 family members [4, 50,51,52,53,54,55,56,57], nucleic acid regulators [58], hypoxia regulators [59] or Bcle family members [60,61,62,63]. Distant disease control includes different long-term endpoints: disease free survival (DFS), overall survival (OS) and distant recurrence rate.
DFS and OS
Survival outcomes have been reported by 5 RCTs, without underling any statistically significant difference, despite the better local disease control [13, 30, 31, 36, 37]. On the contrary, meta-analyses comparing TNT to the standard of care, report homogeneous results regarding DFS improvement in patients undergoing TNT [27, 28, 32, 45, 46]. In particular, the meta-analysis of Riesco-Martinez et al. reported an 18% reduction in risk of recurrence (p = 0.01) and a 19% reduction in mortality at 3 years (p = 0.04) [46].
These results may well be the most important effect of TNT: in fact, traditional neoadjuvant multimodality treatment failed to improve patient survival [64]. Addition of oxaliplatin during standard CRT also had disappointing outcomes [65]. TNT holds the promise of providing tangible, long-term gains prolonging rectal cancer patient lives.
Distant recurrence rate
Distant recurrence rate was reported only in 3 out of 8 RCTs. The GCR-3 and the POLISH II trial did not show any statistically significant difference between the experimental and standard arm [13, 37]. On the contrary, the RAPIDO trial showed a significant reduction in distant recurrence rate in favor of the TNT regimen (67% vs 81%) [30].
Data from 2 meta-analyses showed a reduction by 21,5%-27% in TNT treated patients [27, 46], confirming the RAPIDO trial results [30].
Nevertheless, data are still insufficient to establish whether TNT could improve systemic control disease.
Toxicity/complications
Chemo-related adverse effect
Whether TNT brings a significant rise in neoadjuvant treatment-related adverse effects (AEs) is controversial [66, 67]. Five studies reported AEs rate: four of them showed an increased number of AEs in patients treated with TNT [13, 34, 35, 37] but the PRODIGE-23 showed a statistically significant reduction of adverse events after the administration of neoadjuvant therapy [36].
The most common AEs included diarrhea, nausea, neutropenia and fatigue [28, 32]. All of these and also infectious complications, which were particularly relevant in the RAPIDO trial [30], are reported in the most recent meta-analyses demonstrating the higher risk of developing also serious (Grade III/IV) AEs for TNT patients [32, 45]. Moreover, the CAO/ARO/AIO-12 trial showed grade 3–4 AEs during CRT were more frequent in the induction group compared to the consolidation group [40].
Surgical complications
As reported by almost all the RCTs, TNT does not appear to increase incidence of overall or severe post-operative complications (Clavien-Dindo) [32, 44, 45]. The Memorial Sloan Kettering Center study reported significant benefits such as earlier stoma closure (72% vs 9%) and a 25% higher rate of minimally invasive surgical procedures in TNT treated patients [28, 68]. Moreover, the TIMING trial collected surgeons’ experience, estimating technical difficulty of the operation, that does not seems to differ between different study groups, despite the increased pelvic fibrosis [44].
Compliance
The difference in compliance to TNT and aCT, defined as the administration of at least 75% of the prescribed dose, favor the TNT regimen in most trials. Compliance to TNT ranges from 82 to 100% [44]. In the RAPIDO trial reported compliance to TNT was 84%, consistent with data present in literature, while compliance to CRT and aCT were 93% and 58% respectively. Reasons for not receiving aCT were to be found on difficulties due to surgery but also because of ypN0, pCR or patients’ refusal [30].
Predictors of disease control
Among preoperative prognostic factors, the most important are clinical TNM stage (cTNM), enlarged lateral nodes, extramural vascular invasion, mesorectal fascia involvement and predicted circumferential resection margins (CRM) involvement. Data regarding cTNM reported by all RCTs did not show any difference between experimental and standard arms. Some authors noted how in the RAPIDO trial, tumor regression in terms of ypTNM was not as good with advanced (cT4 tumors) as with other cT stages, suggesting a possible weaker response to TNT by cT4 cancers [69, 70]. However, these considerations are purely speculative as there was no proper cTNM-based stratification.
Unfortunately, extensive data about other preoperative prognostic factors is missing. Five RCTs reported data regarding mesorectal involvement [30, 31, 33, 34, 37] and only one assessed vascular invasion [30]. Predictive CRM [35, 36] and lateral nodes involvement [30, 36] were described by two RCTs. None of these studies reported sub-analysis investigating whether these preoperative factors could be predictive of TNT response. Considering that all kinds of TNT importantly prolong time from diagnosis to radical surgery (which remains the mainstay of rectal cancer curative treatment) and may result in overtreatment for non-responders, it seems reasonable to focus upcoming studies on identifications of factors capable of predicting response to TNT and consequentially to tailor the best treatment for each patient.
Conclusions
CRT seems to be the preferable option in case of high-risk local recurrence cancers. The choice of CT agents other than 5FU or capecitabine seems to be guided only by local policies. Consolidation TNT shows some advantages over induction regimen but differences in long-term survival are still required in order to clarify this highly debated issue. The best timing for surgery following RT is not clear; a minimum of 10 weeks seems an appropriate period to assess pCR and consolidation TNT may allow for extended durations between radiotherapy and surgery. However, timing of surgery apparently does not affect oncological outcomes. TNT increases AEs but does not appear to influence overall survival.
TNT seems to increase pCR and reduce distant recurrence rates. DFS and OS are homogeneously improved with TNT.
Future studies, aimed at evaluating the best regimen, should also investigate factors capable of identifying top responders to TNT in view of a tailored approach for precision oncology treatment.
Availability of data and materials
Not applicable.
Abbreviations
- aCT:
-
Adjuvant Chemotherapy
- AEs:
-
Adverse Effects
- CAPOX/XELOX:
-
Capecitabine and Oxaliplatin
- cCR:
-
Complete Clinical Response
- CRM:
-
Circumferential Resection Margins
- CRT:
-
Chemoradiotherapy
- CT:
-
Chemotherapy
- DFS:
-
Disease Free Survival
- FOLFIRINOX:
-
5FU/Folinic Acid, Irinotecan and Oxaliplatin
- FOLFOX:
-
5FU/Folinic Acid and Oxaliplatin
- LARC:
-
Locally Advanced Rectal Cancer
- LDC:
-
Local Disease Control
- nCT:
-
Neoadjuvant Chemotherapy
- npCR:
-
Near-Complete Pathological Complete Response
- OS:
-
Overall Survival
- pCR:
-
Pathological Complete Response
- PNI:
-
Perineural Invasion
- RCTs:
-
Randomized Clinical Trials
- RT:
-
Radiotherapy
- SCRT:
-
Short-course Radiotherapy
- TNT:
-
Total Neoadjuvant Therapy
- 5FU:
-
5-Fluorouracil
References
Collaborative EuroSurg. Body mass index and complications following major gastrointestinal surgery: a prospective, international cohort study and meta-analysis. Colorectal Dis. 2018;20(8):O215–25. https://doi.org/10.1111/codi.14292.
Fina D, Franze E, Rovedatti L, Corazza GR, Biancone L, Sileri PP, Sica G, MacDonald TT, Pallone F, Di Sabatino A, Monteleone G. Interleukin-25 production is differently regulated by TNF-alpha and TGF-beta 1 in the human gut. Mucosal Immunol. 2011;4(2):239–44. https://doi.org/10.1038/mi.2010.68.
Bucciarelli T, Sacchetta P, Pennelli A, Cornelio L, Romagnoli R, Melino S, Petruzzelli R, Di Ilio C. Characterization of toad liver glutathione transferase. Biochim Biophys Acta. 1999;1431(1):189–98. https://doi.org/10.1016/s0167-4838(99)00036-9.
Nepravishta R, Sabelli R, Iorio E, Micheli L, Paci M, Melino S. Oxidative species and S-glutathionyl conjugates in the apoptosis induction by allyl thiosulfate. FEBS J. 2012;279(1):154–67. https://doi.org/10.1111/j.1742-4658.2011.08407.x.
Liang W, Mo C, Wei J, Chen W, Gong W, Shi J, Hou X, Li C, Deng Y, Ou M. FAM65A as a novel prognostic biomarker in human tumors reveal by a pan-cancer analysis. Discov Oncol. 2021;12(1):60. https://doi.org/10.1007/s12672-021-00456-z.
Lindner AU, Salvucci M, McDonough E, Cho S, Stachtea X, O’Connell EP, Corwin AD, Santamaria-Pang A, Carberry S, Fichtner M, Van Schaeybroeck S, Laurent-Puig P, Burke JP, McNamara DA, Lawler M, Sood A, Graf JF, Rehm M, Dunne PD, Longley DB, Ginty F, Prehn JHM. An atlas of inter- and intra-tumor heterogeneity of apoptosis competency in colorectal cancer tissue at single-cell resolution. Cell Death Differ. 2021. https://doi.org/10.1038/s41418-021-00895-9.
Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D. ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):vi22–40. https://doi.org/10.1093/annonc/mdx224.
Pellino G, Warren O, Mills S, Rasheed S, Tekkis PP, Kontovounisios C. Comparison of Western and Asian Guidelines Concerning the Management of Colon Cancer. Dis Colon Rectum. 2018;61(2):250–9. https://doi.org/10.1097/DCR.0000000000001012.
Chen M, Liu S, Xu M, Yi HC, Liu Y, He F. Radiation boost for synchronous solitary inguinal lymph node metastasis during neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Discov Oncol. 2021;12(1):59. https://doi.org/10.1007/s12672-021-00455-0.
Sun Q, Melino G, Amelio I, Jiang J, Wang Y, Shi Y. Recent advances in cancer immunotherapy. Discov Oncol. 2021;12(1):27. https://doi.org/10.1007/s12672-021-00422-9;).
Heald RJ. The “Holy Plane” of rectal surgery. J R Soc Med. 1988;81(9):503–8.
Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336(14):980–7. https://doi.org/10.1056/NEJM199704033361402.
Bujko K, Wyrwicz L, Rutkowski A, Polish Colorectal Study Group, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27(5):834–42. https://doi.org/10.1093/annonc/mdw062.
Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C, Johansson H, Machado M, Hjern F, Hallböök O, Syk I, Glimelius B, Martling A. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18(3):336–46. https://doi.org/10.1016/S1470-2045(17)30086-4.
Sibio S, Di Giorgio A, D’Ugo S, Palmieri G, Cinelli L, Formica V, Sensi B, Bagaglini G, Di Carlo S, Bellato V, Sica GS. Histotype influences emergency presentation and prognosis in colon cancer surgery. Langenbecks Arch Surg. 2019;404(7):841–51. https://doi.org/10.1007/s00423-019-01826-6.
Sica GS, Fiorani C, Stolfi C, Monteleone G, Candi E, Amelio I, Catani V, Sibio S, Divizia A, Tema G, Iaculli E, Gaspari AL. Peritoneal expression of Matrilysin helps identify early post-operative recurrence of colorectal cancer. Oncotarget. 2015;6(15):13402–15. https://doi.org/10.18632/oncotarget.2830.
Siragusa L, Sensi B, Vinci D, Franceschilli M, Pathirannehalage Don C, Bagaglini G, Bellato V, Campanelli M, Sica GS. Volume-outcome relationship in rectal cancer surgery. Disc Onc. 2021;12(1):11. https://doi.org/10.1007/s12672-021-00406-9.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC, EORTC Radiotherapy Group Trial. EORTC Radiotherapy Group Trial 22921 Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23. https://doi.org/10.1056/NEJMoa060829.
Puhr HC, Wolf P, Berghoff AS, Schoppmann SF, Preusser M, Ilhan-Mutlu A. Elevated free thyroxine levels are associated with poorer overall survival in patients with gastroesophageal cancer: a retrospective single center analysis. Horm Cancer. 2020;11(1):42–51. https://doi.org/10.1007/s12672-019-00374-1.
Amelio I, Bertolo R, Bove P, Candi E, Chiocchi M, Cipriani C, Di Daniele N, Ganini C, Juhl H, Mauriello A, Marani C, Marshall J, Montanaro M, Palmieri G, Piacentini M, Sica G, Tesauro M, Rovella V, Tisone G, Shi Y, Wang Y, Melino G. Cancer predictive studies. Biol Direct. 2020;15(1):18. https://doi.org/10.1186/s13062-020-00274-3.
Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA. Rectal Cancer, Version 2.2018 NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(7):874–901. https://doi.org/10.6004/jnccn.2018.0061.
Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, Marchal D, Van Laethem JL, Klein V, Giralt J, Clavère P, Glanzmann C, Cellier P, Collette L, EORTC Radiation Oncology Group. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184–90. https://doi.org/10.1016/S1470-2045(13)70599-0.
Pellino G, Espín-Basany E. Bowel decontamination before colonic and rectal surgery. Br J Surg. 2021;109(1):3–7. https://doi.org/10.1093/bjs/znab389.
Amelio I, Bertolo R, Bove P, Buonomo O, Candi E, et al. Liquid biopsies and cancer omics. Cell Death Discov. 2020;6(1):131. https://doi.org/10.1038/s41420-020-00373-0.
Bellomaria A, Barbato G, Melino G, Paci M, Melino S. Recognition of p63 by the E3 ligase ITCH: effect of an ectodermal dysplasia mutant. Cell Cycle. 2010;9(18):3730–9.
Lamastra FR, De Angelis R, Antonucci A, Salvatori D, Prosposito P, Casalboni M, Congestri R, Melino S, Nanni F. Polymer composite random lasers based on diatom frustules as scatterers. RSC Adv. 2014;4(106):61809–16. https://doi.org/10.1039/C4RA12519C.
Kong JC, Soucisse M, Michael M, Tie J, Ngan SY, Leong T, McCormick J, Warrier SK, Heriot AG. Total neoadjuvant therapy in locally advanced rectal cancer: a systematic review and metaanalysis of oncological and operative outcomes. Ann Surg Oncol. 2021;28(12):7476–86. https://doi.org/10.1245/s10434-021-09837-8.
Kasi A, Abbasi S, Handa S, Al-Rajabi R, Saeed A, Baranda J, Sun W. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12):e2030097. https://doi.org/10.1001/jamanetworkopen.2020.30097.
Yu H, Huang Y, Ge Y, Hong X, Lin X, Tang K, Wang Q, Yang Y, Sun W, Huang Y, Luo H. Selenite-induced ROS/AMPK/FoxO3a/GABARAPL-1 signaling pathway modulates autophagy that antagonize apoptosis in colorectal cancer cells. Discov Oncol. 2021;12(1):35. https://doi.org/10.1007/s12672-021-00427-4.
Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP, RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42. https://doi.org/10.1016/S1470-2045(20)30555-6.
Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Wu X, Peng J, Ren D, Wang J. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial. J Clin Oncol. 2019;37(34):3223–33. https://doi.org/10.1200/JCO.18.02309.
Liu S, Jiang T, Xiao L, Yang S, Liu Q, Gao Y, Chen G, Xiao W. Total neoadjuvant therapy (TNT) versus standard neoadjuvant chemoradiotherapy for locally advanced rectal cancer: a systematic review and meta-analysis. Oncologist. 2021;26(9):e1555–66. https://doi.org/10.1002/onco.13824.
Moore J, Price T, Carruthers S, Selva-Nayagam S, Luck A, Thomas M, Hewett P. Prospective randomized trial of neoadjuvant chemotherapy during the “wait period” following preoperative chemoradiotherapy for rectal cancer: results of the WAIT trial. Colorectal Dis. 2017;19(11):973–9. https://doi.org/10.1111/codi.13724.
Kim SY, Joo J, Kim TW, Hong YS, Kim JE, Hwang IG, Kim BG, Lee KW, Kim JW, Oh HS, Ahn JB, Zang DY, Kim DY, Oh JH, Baek JY. A randomized phase 2 trial of consolidation chemotherapy after preoperative chemoradiation therapy versus chemoradiation therapy alone for locally advanced rectal cancer: KCSG CO 14-03. Int J Radiat Oncol Biol Phys. 2018;101(4):889–99. https://doi.org/10.1016/j.ijrobp.2018.04.013.
Maréchal R, Vos B, Polus M, Delaunoit T, Peeters M, Demetter P, Hendlisz A, Demols A, Franchimont D, Verset G, Van Houtte P, Van de Stadt J, Van Laethem JL. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23(6):1525–30. https://doi.org/10.1093/annonc/mdr473.
Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardière C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C, Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(5):702–15. https://doi.org/10.1016/S1470-2045(21)00079-6.
Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, Safont MJ, Salud A, Vera R, Massuti B, Escudero P, Alonso V, Bosch C, Martin M, Minsky BD. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26(8):1722–8. https://doi.org/10.1093/annonc/mdv223.
Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL, Schmiegel W, Jacobasch L, Weitz J, Folprecht G, Schlenska-Lange A, Flentje M, Germer CT, Grützmann R, Schwarzbach M, Paolucci V, Bechstein WO, Friede T, Ghadimi M, Hofheinz RD, Rödel C. German rectal cancer study group randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37(34):3212–22. https://doi.org/10.1200/JCO.19.00308.
Garcia-Aguilar J, Patil S, Kim JK, Yuval JB, Thompson H, Verheij F, Lee M, Saltz LB. Behalf of the OPRA Consortium Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 2020;38(15):4008–4008. https://doi.org/10.1200/JCO.2020.38.15_suppl.4008.
Fokas E, Schlenska-Lange A, Polat B, Klautke G, Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL, Kirste S, Jacobasch L, Allgäuer M, Flentje M, Germer CT, Grützmann R, Hildebrandt G, Schwarzbach M, Bechstein WO, Sülberg H, Friede T, Gaedcke J, Ghadimi M, Hofheinz RD, Rödel C. German Rectal Cancer Study Group. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol. 2022;8(1):e215445. https://doi.org/10.1001/jamaoncol.2021.5445.
Lefèvre JH, Mineur L, Cachanado M, Denost Q, Rouanet P, de Chaisemartin C, Meunier B, Mehrdad J, Cotte E, Desrame J, Karoui M, Benoist S, Kirzin S, Berger A, Panis Y, Piessen G, Saudemont A, Prudhomme M, Peschaud F, Dubois A, Loriau J, Tuech JJ, Meurette G, Lupinacci R, Goasguen N, Creavin B, Simon T, Parc Y. The French Research Group of Rectal Cancer Surgery (GRECCAR). Does a longer waiting period after neoadjuvant radio-chemotherapy improve the oncological prognosis of rectal cancer? Three years’ follow-up results of the greccar-6 randomized multicenter trial. Ann Surg. 2019;270(5):747–54. https://doi.org/10.1097/SLA.0000000000003530.
Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, Meunier B, Mehrdad J, Cotte E, Desrame J, Karoui M, Benoist S, Kirzin S, Berger A, Panis Y, Piessen G, Saudemont A, Prudhomme M, Peschaud F, Dubois A, Loriau J, Tuech JJ, Meurette G, Lupinacci R, Goasgen N, Parc Y, Simon T, Tiret E. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol. 2016;34(31):3773–80. https://doi.org/10.1200/JCO.2016.67.6049.
Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90–01 randomized trial. J Clin Oncol. 1999;17:2396.
Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K. Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–66. https://doi.org/10.1016/S1470-2045(15)00004-2.
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–33. https://doi.org/10.1200/JCO.2011.40.1836.
Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, Ghidini M, Turati L. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271(3):440–8. https://doi.org/10.1097/SLA.0000000000003471.
Hou Y, Zhang Q, Pang W, Hou L, Liang Y, Han X, Luo X, Wang P, Zhang X, Li L, Meng X. YTHDC1-mediated augmentation of miR-30d in repressing pancreatic tumorigenesis via attenuation of RUNX1-induced transcriptional activation of Warburg effect. Cell Death Differ. 2021;28(11):3105–24. https://doi.org/10.1038/s41418-021-00804-0.
Mauretti A, Neri A, Kossover O, Seliktar D, Nardo PD, Melino S. Design of a novel composite H2 S-releasing hydrogel for cardiac tissue repair. Macromol Biosci. 2016;16(6):847–58. https://doi.org/10.1002/mabi.201500430.
Melino G, Knight RA, Nicotera P. How many ways to die? How many different models of cell death? Cell Death Differ. 2005;12:1457–62. https://doi.org/10.1038/sj.cdd.4401781.
Rozenberg JM, Zvereva S, Dalina A, Blatov I, Zubarev I, Luppov D, Bessmertnyi A, Romanishin A, Alsoulaiman L, Kumeiko V, Kagansky A, Melino G, Ganini C, Barlev NA. The p53 family member p73 in the regulation of cell stress response. Biol Direct. 2021;16(1):23. https://doi.org/10.1186/s13062-021-00307-5.
Candi E, Agostini M, Melino G, Bernassola F. How the TP53 family proteins TP63 and TP73 contribute to tumorigenesis: regulators and effectors. Hum Mutat. 2014;35(6):702–14. https://doi.org/10.1002/humu.22523.
Tomasini R, Tsuchihara K, Tsuda C, Lau SK, Wilhelm M, Rufini A, Tsao MS, Iovanna JL, Jurisicova A, Melino G, Mak TW. TAp73 regulates the spindle assembly checkpoint by modulating BubR1 activity. Proc Natl Acad Sci U S A. 2009;106(3):797–802. https://doi.org/10.1073/pnas.0812096106.
De Laurenzi V, Melino G. Evolution of functions within the p53/p63/p73 family. Ann N Y Acad Sci. 2000;926:90–100. https://doi.org/10.1111/j.1749-6632.2000.tb05602.x.
Panatta E, Zampieri C, Melino G, Amelio I. Understanding p53 tumour suppressor network. Biol Direct. 2021;16(1):14. https://doi.org/10.1186/s13062-021-00298-3.
Bernassola F, Salomoni P, Oberst A, Di Como CJ, Pagano M, Melino G, Pandolfi PP. Ubiquitin-dependent degradation of p73 is inhibited by PML. J Exp Med. 2004;199(11):1545–57. https://doi.org/10.1084/jem.20031943.
Gallo M, Paludi D, Cicero DO, Chiovitti K, Millo E, Salis A, Damonte G, Corsaro A, Thellung S, Schettini G, Melino S, Florio T, Paci M, Aceto A. Identification of a conserved N-capping box important for the structural autonomy of the prion alpha 3-helix: the disease associated D202N mutation destabilizes the helical conformation. Int J Immunopathol Pharmacol. 2005;18(1):95–112. https://doi.org/10.1177/039463200501800111.
Mammarella E, Zampieri C, Panatta E, Melino G, Amelio I. NUAK2 and RCan2 participate in the p53 mutant pro-tumorigenic network. Biol Direct. 2021;16(1):11. https://doi.org/10.1186/s13062-021-00296-5.
Melino S, Nepravishta R, Bellomaria A, Di Marco S, Paci M. Nucleic acid binding of the RTN1-C C-terminal region: toward the functional role of a reticulon protein. Biochemistry. 2009;48(2):242–53. https://doi.org/10.1021/bi801407w.
Amelio I, Melino G. The p53 family and the hypoxia-inducible factors (HIFs): determinants of cancer progression. Trends Biochem Sci. 2015;40(8):425–34. https://doi.org/10.1016/j.tibs.2015.04.007.
Dengler MA, Gibson L, Adams JM. BAX mitochondrial integration is regulated allosterically by its α1-α2 loop. Cell Death Differ. 2021;28(12):3270–81. https://doi.org/10.1038/s41418-021-00815-x.
Ramesh P, Lannagan TRM, Jackstadt R, Atencia Taboada L, Lansu N, Wirapati P, van Hooff SR, Dekker D, Pritchard J, Kirov AB, van Neerven SM, Tejpar S, Kops GJPL, Sansom OJ, Medema JP. BCL-XL is crucial for progression through the adenoma-to-carcinoma sequence of colorectal cancer. Cell Death Differ. 2021;28(12):3282–96. https://doi.org/10.1038/s41418-021-00816-w.
Wang X, Ros U, Agrawal D, Keller EC, Slotta-Huspenina J, Dill V, Shen B, Shi R, Herold T, Belka C, Mishra R, Bassermann F, Garcia-Saez AJ, Jost PJ. MLKL promotes cellular differentiation in myeloid leukemia by facilitating the release of G-CSF. Cell Death Differ. 2021;28(12):3235–50. https://doi.org/10.1038/s41418-021-00811-1.
La Montagna M, Shi L, Magee P, Sahoo S, Fassan M, Garofalo M. AMPKα loss promotes KRAS-mediated lung tumorigenesis. Cell Death Differ. 2021;28(9):2673–89. https://doi.org/10.1038/s41418-021-00777-0.
Riesco-Martinez MC, Fernandez-Martos C, Gravalos-Castro C, Espinosa-Olarte P, La Salvia A, Robles-Diaz L, Modrego-Sanchez A, Garcia-Carbonero R. Impact of total neoadjuvant therapy vs. standard chemoradiotherapy in locally advanced rectal cancer: a systematic review and meta-analysis of randomized trials. Cancers (Basel). 2020;12(12):3655. https://doi.org/10.3390/cancers12123655.
Roede C. Short-course Radiotherapy Versus Chemoradiotherapy, Followed by Consolidation Chemotherapy, and Selective Organ Preservation for MRI-defined Intermediate and High-risk Rectal Cancer Patients. [accessed 2020 Jan 29]. In: ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04246684#contacts ClinicalTrials.gov Identifier: NCT04246684
Zheng J, Feng X, Hu W, Wang J, Li Y. Systematic review and meta-analysis of preoperative chemoradiotherapy with or without oxaliplatin in locally advanced rectal cancer. Medicine (Baltimore). 2017;96(13):e6487. https://doi.org/10.1097/MD.0000000000006487.
Espin Basany E, Solís-Peña A, Pellino G, Kreisler E, Fraccalvieri D, Muinelo-Lorenzo M, Maseda-Díaz O, García-González JM, Santamaría-Olabarrieta M, Codina-Cazador A, Biondo S. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(8):729–38. https://doi.org/10.1016/S2468-1253(20)30075-3.
Pellino G, Sciaudone G, Candilio G, Camerlingo A, Marcellinaro R, De Fatico S, Rocco F, Canonico S, Riegler G, Selvaggi F. Early postoperative administration of probiotics versus placebo in elderly patients undergoing elective colorectal surgery: a double-blind randomized controlled trial. BMC Surg. 2013;13(Suppl 2):S57. https://doi.org/10.1186/1471-2482-13-S2-S57.
Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, Guillem JG, Paty PB, Yaeger R, Stadler ZK, Seier K, Gonen M, Segal NH, Reidy DL, Varghese A, Shia J, Vakiani E, Wu AJ, Crane CH, Gollub MJ, Garcia-Aguilar J, Saltz LB, Weiser MR. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4(6):e180071. https://doi.org/10.1001/jamaoncol.2018.0071.
Glynne-Jones R. Interpreting the RAPIDO trial: factors to consider. Lancet Oncol. 2021;22(3):e85. https://doi.org/10.1016/S1470-2045(21)00013-9].
Acknowledgements
Not applicable.
Funding
This research did not receive grants from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
AMG, BS and GSS collected ideas and contributions and did most of the writing; VF, RMDA, MR, GDVB, PR, GTC and MC gave substantial contributions to conception and design of the study and data interpretation; AMG and GSS revised the manuscript. All authors acccepted the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The Authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guida, A.M., Sensi, B., Formica, V. et al. Total neoadjuvant therapy for the treatment of locally advanced rectal cancer: a systematic minireview. Biol Direct 17, 16 (2022). https://doi.org/10.1186/s13062-022-00329-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13062-022-00329-7