Abstract
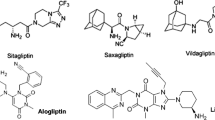
The worldwide incidence of type 2 diabetes mellitus (T2DM), a chronic metabolic disorder, is increasing rapidly. A newer category of oral hypoglycemic medications called dipeptidyl peptidases-4 (DPP-4) inhibitors has come into existence for the management of T2DM. DPP-4 inhibitors, i.e., linagliptin, saxagliptin, and sitagliptin, belong to BCS class III, while alogliptin belongs to BCS class I. These drugs have high aqueous solubility and, therefore, short elimination half-life which necessitates the need for multiple daily dosing. Furthermore, the unrestrained drug release from conventional tablets can cause elevation in systemic drug concentrations which might instigate gastrointestinal side effects. The poor membrane permeability of BCS class III drugs also results in their poor oral bioavailability. Therefore, clinical compliance and therapeutic efficacy of DPP-4 inhibitors can be enhanced by nanotechnology-based techniques. This research paper has described various risk factors and pathophysiology of T2DM and also explained about the mode of action of DPP-4 inhibitors. The main objectives and rationale of this review include the exploration of preclinical pharmacological investigations and the summarization of developed nanoformulations of DPP-4 inhibitors researched in previous decades. The nanoformulations which has been synthesized for DPP-4 inhibitors in the past few decades for the management of T2DM include polymeric nanoparticle, solid lipid nanoparticle, transferosomes, niosomes, mucoadhesive nanoparticle, and self-microemulsifying drug delivery systems.



Similar content being viewed by others
Availability of Data and Materials
Not applicable.
References
Kaul, K., Tarr, J.M., Ahmad, S.I., Kohner, E.M., & Chibber, R. (2013). Introduction to diabetes mellitus. In S. I. Ahmad (Ed.), Diabetes. Advances in Experimental Medicine and Biology (vol. 771). Springer.
Grewal, A. S., Thapa, K., Kanojia, N., Sharma, N., & Singh, S. (2020). Natural compounds as source of aldose reductase (Ar) inhibitors for the treatment of diabetic complications: A mini review. Current Drug Metabolism, 21(14), 1091–1116.
Andukuri, R., Drincic, A., & Rendell, M. (2009). Alogliptin: A new addition to the class of DPP-4 inhibitors. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 2, 117–126.
Forouhi, N. G., & Wareham, N. J. (2010). Epidemiology of diabetes. Medicine, 38(11), 602–606.
Mayer-Davis, E. J., Kahkoska, A. R., Jefferies, C., Dabelea, D., Balde, N., Gong, C. X., Aschner, P., & Craig, M. E. (2018). ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric Diabetes, 19(Suppl 27), 7–19.
Bobiş, O., Dezmirean, D. S., & Moise, A. R. (2018). Honey and diabetes: The importance of natural simple sugars in diet for preventing and treating different type of diabetes. Oxidative Medicine and Cellular Longevity, 2018, 1–12.
Makrilakis, K. (2019). The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: When to select, what to expect. International Journal of Environmental Research and Public Health, 16(15), 2720.
Keating, G. M. (2015). Alogliptin: A review of its use in patients with type 2 diabetes mellitus. Drugs, 75, 777–796.
de Abreu Engel, R. E., Barden, A. T., Campanharo, S. C., Olegário, N., Volpato, N. M., & Schapoval, E. E. S. (2019). Evaluation of linagliptin dissolution from tablets using HPLC and UV methods. Drug Analytical Research, 3(2), 46–50.
Lyseng-Williamson, K. A. (2007). Sitagliptin. Drugs, 67, 587–597.
Wondmkun, Y. T. (2020). Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes, Metabolic Syndrome and Obesity, 13, 3611–3616.
Ozougwu, J. C., Obimba, K. C., Belonwu, C. D., & Unakalamba, C. B. (2013). The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. Journal of Physiology and Pathophysiology, 4(4), 46–57.
Dendup, T., Feng, X., Clingan, S., & Astell-Burt, T. (2018). Environmental risk factors for developing type 2 diabetes mellitus: A systematic review. International Journal of Environmental Research and Public Health, 15(1), 78.
Harding, J. L., Pavkov, M. E., Magliano, D. J., Shaw, J. E., & Gregg, E. W. (2019). Global trends in diabetes complications: A review of current evidence. Diabetologia, 62, 3–16.
Mansour, A., Mousa, M., Abdelmannan, D., Tay, G., Hassoun, A., & Alsafar, H. (2023). Microvascular and macrovascular complications of type 2 diabetes mellitus: Exome wide association analyses. Frontiers in Endocrinology, 14, 1143067.
Rahman, S., Rahman, T., Ismail, A. A., & Rashid, A. R. A. (2007). Diabetes-associated macrovasculopathy: Pathophysiology and pathogenesis. Diabetes, Obesity and Metabolism, 9(6), 767–780.
Oh, J.-W., Muthu, M., Haga, S. W., Anthonydhason, V., Paul, P., & Chun, S. (2020). Reckoning the dearth of bioinformatics in the arena of diabetic nephropathy (DN)—need to improvise. Processes, 8(7), 808.
Lankatillake, C., Huynh, T., & Dias, D. A. (2019). Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods, 15(1), 1–35.
Beulens, J. W. J., Pinho, M. G. M., Abreu, T. C., den Braver, N. R., Lam, T. M., Huss, A., Vlaanderen, J., Sonnenschein, T., Siddiqui, N. Z., Yuan, Z., Kerckhoffs, J., Zhernakova, A., Gois, M. F. B., & Vermeulen, R. C. H. (2021). Environmental risk factors of type 2 diabetes-an exposome approach. Diabetologia, 65, 263–274.
Association, A. D. (2010). Diagnosis and classification of diabetes mellitus. Diabetes care, 33(Supplement_1), S62–S69.
Khan, M. A. B., Hashim, M. J., King, J. K., Govender, R. D., Mustafa, H., & Al Kaabi, J. (2020). Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. Journal of Epidemiology and Global Health, 10(1), 107.
Nithya, V., Sangavi, P., Srinithi, R., Nachammai, K. T., Gowtham Kumar, S., Prabu, D., & Langeswaran, K. (2023). Diabetes and other comorbidities: Microvascular and macrovascular diseases diabetes and cancer. In Rana Noor (Ed.), Advances in Diabetes Research and Management (pp. 21–39). Springer.
Baynes, H. W. (2015). Classification, pathophysiology, diagnosis and management of diabetes mellitus. Journal of Diabetes & Metabolism, 6(5), 1–9.
Glovaci, D., Fan, W., & Wong, N. D. (2019). Epidemiology of diabetes mellitus and cardiovascular disease. Current Cardiology Reports, 21, 1–8.
Burn, P. (2010). Type 1 diabetes. Nature Reviews Drug discovery, 9(3), 187.
Cooke, D. W., & Plotnick, L. (2008). Type 1 diabetes mellitus in pediatrics. Pediatrics in Review, 29(11), 374–385.
Boles, A., Kandimalla, R., & Reddy, P. H. (2017). Dynamics of diabetes and obesity: Epidemiological perspective. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1863(5), 1026–1036.
Galicia-Garcia, U., Benito-Vicente, A., Jebari, S., Larrea-Sebal, A., Siddiqi, H., Uribe, K. B., Ostolaza, H., & Martín, C. (2020). Pathophysiology of type 2 diabetes mellitus. International Journal of Molecular Sciences, 21(17), 6275.
Olokoba, A. B., Obateru, O. A., & Olokoba, L. B. (2012). Type 2 diabetes mellitus: A review of current trends. Oman Medical Journal, 27(4), 269.
Stumvoll, M., Goldstein, B. J., & Van Haeften, T. W. (2005). Type 2 diabetes: Principles of pathogenesis and therapy. The Lancet, 365(9467), 1333–1346.
DeFronzo, R. A., Ferrannini, E., Groop, L., Henry, R. R., Herman, W. H., Holst, J. J., Hu, F. B., Kahn, C. R., Raz, I., Shulman, G. I., & Simonson, D. C. (2015). Type 2 diabetes mellitus. Nature reviews Disease Primers, 1(1), 1–22.
Chiefari, E., Arcidiacono, B., Foti, D., & Brunetti, A. (2017). Gestational diabetes mellitus: An updated overview. Journal of Endocrinological Investigation, 40, 899–909.
Johns, E. C., Denison, F. C., Norman, J. E., & Reynolds, R. M. (2018). Gestational diabetes mellitus: Mechanisms, treatment, and complications. Trends in Endocrinology & Metabolism, 29(11), 743–754.
McIntyre, H. D., Catalano, P., Zhang, C., Desoye, G., Mathiesen, E. R., & Damm, P. (2019). Gestational diabetes mellitus. Nature Reviews Disease Primers, 5(1), 47.
Ahmad, K. (2014). Insulin sources and types: A review of insulin in terms of its mode on diabetes mellitus. Journal of Traditional Chinese Medicine, 34(2), 234–237.
Bastaki, S. (2005). Diabetes mellitus and its treatment. Dubai Diabetes and Endocrinology Journal, 13(3), 111–134.
Zahoor, I., Singh, S., Behl, T., Sharma, N., Naved, T., Subramaniyan, V., & Al-Harrasi, A. (2022). Emergence of microneedles as a potential therapeutics in diabetes mellitus. Environmental Science and Pollution Research, 29, 3302–3322.
Grarup, N., Sandholt, C. H., Hansen, T., & Pedersen, O. (2014). Genetic susceptibility to type 2 diabetes and obesity: From genome-wide association studies to rare variants and beyond. Diabetologia, 57, 1528–1541.
Zaccardi, F., Webb, D. R., Yates, T., & Davies, M. J. (2016). Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgraduate Medical Journal, 92(1084), 63–69.
Hu, F. B., Manson, J. E., Stampfer, M. J., Colditz, G., Liu, S., Solomon, C. G., & Willett, W. C. (2001). Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. New England Journal of Medicine, 345(11), 790–797.
Schellenberg, E. S., Dryden, D. M., Vandermeer, B., Ha, C., & Korownyk, C. (2013). Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Annals of Internal Medicine, 159(8), 543–551.
Andersen, E. S., Deacon, C. F., & Holst, J. J. (2018). Do we know the true mechanism of action of the DPP-4 inhibitors? Diabetes, Obesity and Metabolism, 20(1), 34–41.
Istrate, D., & Crisan, L. (2022). Natural compounds as DPP-4 inhibitors: 3D-similarity search, ADME toxicity, and molecular docking approaches. Symmetry, 14(9), 1842.
Mathur, V., Alam, O., Siddiqui, N., Jha, M., Manaithiya, A., Bawa, S., Sharma, N., Alshehri, S., Alam, P., & Shakeel, F. (2023). Insight into structure activity relationship of DPP-4 inhibitors for development of antidiabetic agents. Molecules, 28(15), 5860.
Cada, D. J., Levien, T. L., & Baker, D. E. (2013). Alogliptin. Hospital Pharmacy, 48(7), 580–592.
Boddu, R., Vadla, H. C., Prathap, V. R., Kothamasu, U., Rallabandi, B. C., & Gannu, R. (2021). Development of an in vitro-in vivo correlation for sitagliptin and metformin prolonged-release tablet formulations. Turkish Journal of Pharmaceutical Sciences, 18(2), 233.
Shrestha, N., Araujo, F., Shahbazi, M.-A., Mäkilä, E., Gomes, M. J., Airavaara, M., Kauppinen, E. I., Raula, J., Salonen, J., Hirvonen, J., & Sarmento, B. (2016). Oral hypoglycaemic effect of GLP-1 and DPP4 inhibitor based nanocomposites in a diabetic animal model. Journal of Controlled Release, 232, 113–119.
Thondawada, M., Wadhwani, A. D., S. Palanisamy, D., Rathore, H. S., Gupta, R. C., Chintamaneni, P. K., Samanta, M.K., Dubala, A., Varma, S., Krishnamurthy, P.T., & Gowthamarajan, K. (2018). An effective treatment approach of DPP-IV inhibitor encapsulated polymeric nanoparticles conjugated with anti-CD-4 mAb for type 1 diabetes. Drug Development and Industrial Pharmacy, 44(7), 1120–1129.
Dobnig, H., & Amrein, K. (2019). Best practice & research clinical endocrinology & metabolism.
Dhillon, S. (2010). Sitagliptin: A review of its use in the management of type 2 diabetes mellitus. Drugs, 70, 489–512.
Dave, D. J. (2011). Saxagliptin: A dipeptidyl peptidase-4 inhibitor in the treatment of type 2 diabetes mellitus. Journal of Pharmacology and Pharmacotherapeutics, 2(4), 230–235.
Rasul, A., Maheen, S., Khan, H. U., Rasool, M., Shah, S., Abbas, G., Afzal, K., Tariq, F., Shahzadi, I., & Asad, M. H. H. B. (2021). Formulation, optimization, in vitro and in vivo evaluation of saxagliptin-loaded lipospheres for an improved pharmacokinetic behavior. BioMed Research International, 2021, 1–17.
Plosker, G. L. (2014). Sitagliptin: A review of its use in patients with type 2 diabetes mellitus. Drugs, 74(2), 223–242.
Scott, L. J. (2017). Sitagliptin: A review in type 2 diabetes. Drugs, 77, 209–224.
Lyseng-Williamson, K. A., & Yang, L. P. H. (2014). Saxagliptin: A guide to its use in type 2 diabetes mellitus. Drugs & Therapy Perspectives, 30(3), 92–99.
Scheen, A. J. (2010). Pharmacokinetics of dipeptidylpeptidase-4 inhibitors. Diabetes, Obesity and Metabolism, 12(8), 648–658.
Kania, D. S., Gonzalvo, J. D., & Weber, Z. A. (2011). Saxagliptin: A clinical review in the treatment of type 2 diabetes mellitus. Clinical Therapeutics, 33(8), 1005–1022.
Dhillon, S., & Weber, J. (2009). Saxagliptin. Drugs, 69, 2103–2114.
Scott, L. J. (2011). Linagliptin: In type 2 diabetes mellitus. Drugs, 71, 611–624.
Graefe-Mody, U., Retlich, S., & Friedrich, C. (2012). Clinical pharmacokinetics and pharmacodynamics of linagliptin. Clinical Pharmacokinetics, 51, 411–427.
Deeks, E. D. (2012). Linagliptin: A review of its use in the management of type 2 diabetes mellitus. Drugs, 72, 1793–1824.
White, W. B., Cannon, C. P., Heller, S. R., Nissen, S. E., Bergenstal, R. M., Bakris, G. L., Perez, A. T., Fleck, P. R., Mehta, C. R., Kupfer, S., & Wilson, C. (2013). Alogliptin after acute coronary syndrome in patients with type 2 diabetes. New England Journal of Medicine, 369(14), 1327–1335.
Kaku, K., Kisanuki, K., Shibata, M., & Oohira, T. (2019). Benefit-risk assessment of alogliptin for the treatment of type 2 diabetes mellitus. Drug Safety, 42, 1311–1327.
Waget, A., Cabou, C., Masseboeuf, M., Cattan, P., Armanet, M., Karaca, M., Castel, J., Garret, C., Payros, G., Maida, A., & Sulpice, T. (2011). Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology, 152(8), 3018–3029.
Eggadi, V., Sheshagiri, S. B. B., Devandla, A., Dasi, N., Kulundaivelu, U., Revoori, S. K., Kesireddy, S. R., Revoori, K., & Keshireddy, S. R. (2015). Effect of atorvastatin on pharmacology of sitagliptin in streptozotocin-nicotinamide induced type-II diabetes in rats. Biology and Medicine, 7(1), 1.
Eitah, H. E., Maklad, Y. A., Abdelkader, N. F., El Din, A. A. G., Badawi, M. A., & Kenawy, S. A. (2019). Modulating impacts of quercetin/sitagliptin combination on streptozotocin-induced diabetes mellitus in rats. Toxicology and Applied Pharmacology, 365, 30–40.
Samaha, M. M., Said, E., & Salem, H. A. (2019). A comparative study of the role of crocin and sitagliptin in attenuation of STZ-induced diabetes mellitus and the associated inflammatory and apoptotic changes in pancreatic β-islets. Environmental Toxicology and Pharmacology, 72, 103238.
Craig, S. L., Gault, V. A., Flatt, P. R., & Irwin, N. (2021). The methionine aminopeptidase 2 inhibitor, TNP-470, enhances the antidiabetic properties of sitagliptin in mice by upregulating xenin. Biochemical Pharmacology, 183, 114355.
Hou, J., Zheng, D., Fan, K., Yu, B., Xiao, W., Ma, J., Jin, W., Tan, Y., & Wu, J. (2012). Combination of mangiferin and dipeptidyl peptidase-4 inhibitor sitagliptin improves impaired glucose tolerance in streptozotocin-diabetic rats. Pharmacology, 90(3–4), 177–182.
Wang, J., Hu, L., Chen, Y., Fu, T., Jiang, T., Jiang, A., & You, X. (2019). Sitagliptin improves renal function in diabetic nephropathy in male Sprague Dawley rats through upregulating heme oxygenase-1 expression. Endocrine, 63, 70–78.
Karabulut, S., Coskun, Z. M., & Bolkent, S. (2015). Immunohistochemical, apoptotic and biochemical changes by dipeptidyl peptidase-4 inhibitor-sitagliptin in type-2 diabetic rats. Pharmacological Reports, 67(5), 846–853.
Al-Damry, N. T., Attia, H. A., Al-Rasheed, N. M., Al-Rasheed, N. M., Mohamad, R. A., Al-Amin, M. A., Dizmiri, N., & Atteya, M. (2018). Sitagliptin attenuates myocardial apoptosis via activating LKB-1/AMPK/Akt pathway and suppressing the activity of GSK-3β and p38α/MAPK in a rat model of diabetic cardiomyopathy. Biomedicine & Pharmacotherapy, 107, 347–358.
Reimer, R. A., Grover, G. J., Koetzner, L., Gahler, R. J., Juneja, P., Lyon, M. R., & Wood, S. (2012). Sitagliptin reduces hyperglycemia and increases satiety hormone secretion more effectively when used with a novel polysaccharide in obese Zucker rats. The Journal of Nutrition, 142(10), 1812–1820.
Chang, Y., Sun, B., Han, Z., Han, F., Hu, S., Li, X., Xue, M., Yang, Y., Chen, L., Li, C. J., & Chen, L. M. (2017). Saxagliptin attenuates albuminuria by inhibiting podocyte epithelial-to-mesenchymal transition via SDF-1α in diabetic nephropathy. Frontiers in Pharmacology, 8, 780.
Schürmann, C., Linke, A., Engelmann-Pilger, K., Steinmetz, C., Mark, M., Pfeilschifter, J., Klein, T., & Frank, S. (2012). The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. Journal of Pharmacology and Experimental Therapeutics, 342(1), 71–80.
Dietrich, N., Kolibabka, M., Busch, S., Bugert, P., Kaiser, U., Lin, J., Fleming, T., Morcos, M., Klein, T., Schlotterer, A., & Hammes, H.P. (2016). The DPP4 inhibitor linagliptin protects from experimental diabetic retinopathy. PloS one, 11(12), e0167853
Nakamura, Y., Inagaki, M., Shimizu, T., Fujita, K., Inoue, M., Gotoh, H., Oguchi, K., & Goto, Y. (2013). Long-term effects of alogliptin benzoate in hemodialysis patients with diabetes: A 2-year study. Nephron Clinical Practice, 123(1–2), 46–51.
Jahangir, M. A., Khan, R., & Sarim Imam, S. (2018). Formulation of sitagliptin-loaded oral polymeric nano scaffold: Process parameters evaluation and enhanced anti-diabetic performance. Artificial Cells, Nanomedicine, and Biotechnology, 46(sup1), 66–78.
Harsha, S., Attimard, M., Khan, T. A., Nair, A. B., Aldhubiab, B. E., Sangi, S., & Shariff, A. (2013). Design and formulation of mucoadhesive microspheres of sitagliptin. Journal of Microencapsulation, 30(3), 257–264.
Kazi, M., Alqahtani, A., Ahmad, A., Noman, O. M., Aldughaim, M. S., Alqahtani, A. S., & Alanazi, F. K. (2021). Development and optimization of sitagliptin and dapagliflozin loaded oral self-nanoemulsifying formulation against type 2 diabetes mellitus. Drug Delivery, 28(1), 100–114.
SreeHarsha, N., Ramnarayanan, C., Al-Dhubiab, B. E., Nair, A. B., Hiremath, J. G., Venugopala, K. N., Satish, R.T., Attimarad, M., & Shariff, A. (2019). Mucoadhesive particles: A novel, prolonged-release nanocarrier of sitagliptin for the treatment of diabetics. BioMed Research International, 2019.
Prabahar, K., Udhumansha, U., & Qushawy, M. (2020). Optimization of thiolated chitosan nanoparticles for the enhancement of in vivo hypoglycemic efficacy of sitagliptin in streptozotocin-induced diabetic rats. Pharmaceutics, 12(4), 300.
Nair, A. B., Sreeharsha, N., Al-Dhubiab, B. E., Hiremath, J. G., Shinu, P., Attimarad, M., Venugopala, K. N., & Mutahar, M. (2019). HPMC-and PLGA-based nanoparticles for the mucoadhesive delivery of sitagliptin: Optimization and in vivo evaluation in rats. Materials, 12(24), 4239.
HaqAsif, A., Harsha, S., HodalurPuttaswamy, N., & Al-Dhubiab, E. B. (2018). An effective delivery system of sitagliptin using optimized mucoadhesive nanoparticles. Applied Sciences, 8(6), 861.
Rachel, K. F. (2022). Development and characterization of anti-diabetic liposomal formulation. International Journal of Green Pharmacy (IJGP), 16(1).
Alhamhoom, Y., Ravi, G., Osmani, R. A. M., Hani, U., & Prakash, G. M. (2022). Formulation, characterization, and evaluation of eudragit-coated saxagliptin nanoparticles using 3 factorial design modules. Molecules, 27(21), 7510.
Maheen, S., Rasul, A., Hanif, M., & Khan, H. U. (2020). Lipospheres for simultaneous controlled release and improved pharmacokinetic profiles of saxagliptin-enalapril: Formulation, optimization, and comparative in vitro-in vivo evaluation. An Official Journal of the American Association of Pharmaceutical Scientists, 21, 1–16.
Shah, P., Chavda, K., Vyas, B., & Patel, S. (2021). Formulation development of linagliptin solid lipid nanoparticles for oral bioavailability enhancement: Role of P-gp inhibition. Drug Delivery and Translational Research, 11, 1166–1185.
Veni, D. K., & Gupta, N. V. (2020). Development and evaluation of Eudragit coated environmental sensitive solid lipid nanoparticles using central composite design module for enhancement of oral bioavailability of linagliptin. International Journal of Polymeric Materials and Polymeric Biomaterials, 69(7), 407–418.
Navaneetha, K., Navya, A., Venkateshwara, B., Reddy, T., & Saritha, N. J. (2017). Formulation and in-vitro evaluation of nanoparticles of linagliptin. WJPR, 6(7), 1319–1328.
Nishu, S. B. N., Karmoker, J. R., Ali, F. F., Rafa, N. N., Hoque, O., & Dewan, I. (2018). In vitro and ex vivo studies of linagliptin loaded non-ionic surfactant vesicles using statistical optimization. Journal of Advances in Medical and Pharmaceutical Sciences, 18(2), 1–16.
Rahi, F. A., Ameen, M. S. M., & Fayyadh, M. S. (2021). Linagliptin and gliclazide di-loaded extended-release nanoparticles: Formulation and evaluation. Wiadomosci Lekarskie (Warsaw, Poland: 1960), 74(9 cz 2), 2315–2322.
Mohanty, D., Gilani, S. J., Zafar, A., Imam, S. S., Kumar, L. A., Ahmed, M. M., Jahangir, M. A., Bakshi, V., Ahmad, W., & Eltayib, E. M. (2022). Formulation and optimization of alogliptin-loaded polymeric nanoparticles: In vitro to in vivo assessment. Molecules, 27(14), 4470.
Nagavarma, B. V. N., Yadav, H. K. S., Ayaz, A., Vasudha, L. S., & Shivakumar, H. G. (2012). Different techniques for preparation of polymeric nanoparticles-a review. Asian Journal of Pharmaceutical and Clinical Research, 5(3), 16–23.
Crucho, C. I. C., & Barros, M. T. (2017). Polymeric nanoparticles: A study on the preparation variables and characterization methods. Materials Science and Engineering: C, 80, 771–784.
Rao, J. P., & Geckeler, K. E. (2011). Polymer nanoparticles: Preparation techniques and size-control parameters. Progress in Polymer Science, 36(7), 887–913.
Mukherjee, S., Ray, S., & Thakur, R. S. (2009). Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian Journal of Pharmaceutical Sciences, 71(4), 349.
Ganesan, P., & Narayanasamy, D. (2017). Lipid nanoparticles: Different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustainable Chemistry and Pharmacy, 6, 37–56.
Chauhan, N., Kumar, K., & Pant, N. C. (2017). An updated review on transfersomes: A novel vesicular system for transdermal drug delivery. Universal Journal of Pharmaceutical Research, 2(4), 42–45.
Rai, S., Pandey, V., & Rai, G. (2017). Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Reviews & Experiments, 8(1), 1325708.
Solanki, D., Kushwah, L., Motiwale, M., & Chouhan, V. (2016). Transferosomes-a review. World Journal of Pharmacy and Pharmaceutical Sciences, 5(10), 435–449.
Bhardwaj, P., Tripathi, P., Gupta, R., & Pandey, S. (2020). Niosomes: A review on niosomal research in the last decade. Journal of Drug Delivery Science and Technology, 56, 101581.
Yeo, P. L., Lim, C. L., Chye, S. M., Ling, A. P. K., & Koh, R. Y. (2017). Niosomes: A review of their structure, properties, methods of preparation, and medical applications. Asian Biomedicine, 11(4), 301–314.
Tangri, P., & Khurana, S. (2011). Niosomes: Formulation and evaluation. International Journal of Biopharmaceutics, 2(1), 47–53.
Moghassemi, S., & Hadjizadeh, A. (2014). Nano-niosomes as nanoscale drug delivery systems: An illustrated review. Journal of Controlled Release, 185, 22–36.
Das Neves, J., Bahia, M. F., Amiji, M. M., & Sarmento, B. (2011). Mucoadhesive nanomedicines: Characterization and modulation of mucoadhesion at the nanoscale. Expert Opinion on Drug Delivery, 8(8), 1085–1104.
Sapre, A. S., & Parikh, R. K. (2012). Design of a buccal mucoadhesive, nanoparticles based delivery system of fluoxetine. JPSBR, 2(3), 148–161.
Takeuchi, H., Yamamoto, H., & Kawashima, Y. (2001). Mucoadhesive nanoparticulate systems for peptide drug delivery. Advanced Drug Delivery Reviews, 47(1), 39–54.
Jaiswal, P., & Aggarwal, G. (2013). Bioavailability enhancdement of poorly soluble drugs by smedds: A review. Journal of Drug Delivery and Therapeutics, 3(1).
Dokania, S., & Joshi, A. K. (2015). Self-microemulsifying drug delivery system (SMEDDS)–challenges and road ahead. Drug Delivery, 22(6), 675–690.
Potphode, V. R., Deshmukh, A. S., & Mahajan, V. R. (2016). Self-micro emulsifying drug delivery system: An approach for enhancement of bioavailability of poorly water soluble drugs. Asian Journal of Pharmacy and Technology, 6(3), 159–168.
Maurya, S. D., Arya, R. K. K., Rajpal, G., & Dhakar, R. C. (2017). Self-micro emulsifying drug delivery systems (SMEDDS): A review on physico-chemical and biopharmaceutical aspects. Journal of Drug Delivery and Therapeutics, 7(3), 55–65.
Ersin, Y., Bayram, K., Fatma, Ö., Tansel, A., & Celil, Ü. (2022). Oral formulations comprising sitagliptin HCI monohydrate with improved pharmaceutical characteristics. EP4045048.
Xufeng, W. U., Li, S., Dadong, S., Pengcheng, L. I. U., Haoling, G. A. O., Dengfeng, D., & Lingling, W. (2022). Purification method of sitagliptin intermediate. CN114644568.
Anthony, R., & Stephen, M. (2022). Low-dose triple combination formulation. US20220184070.
Hong, G. U. O., Hai, G. U. O., & Kelin, S. H. I. (2022). Chemical treatment device for sitagliptin phosphate raw material. CN216677141.
Hong, G. U. O., Hai, G. U. O., & Kelin, S. H. I. (2022). Sitagliptin phosphate medicine raw material treatment and extraction device. CN216653466.
Seval, A., & Muge, U. B. (2022). An effervescent tablet composition of sitagliptin. EP3999070.
Christou, K. E., Christou, K. I., Leonida, K. A., Christou, S. V., Konstantinou, K. A., Andrea, K. E., & Stylianou, F. M. (2022). Pharmaceutical composition comprising a combination of sitagliptin and metformin and method of preparation thereof. GR1010234.
Hui, L. I. U. (2022). Preparation method of low-cost sitagliptin phosphate. CN11450769.
Sathyanarayana, V., & Pankaj, P. (2022). An immediate release composition of sitagliptin hydrochloride. WO2022074664.
Evangelos, K., Efthymios, K., Vasiliki, S., Ioanna, K., Anastasia, K., Andreas, K., & Manolis, F. (2022). Solid dosage form comprising sitagliptin and method of preparation thereof. WO2022058044.
Xiang, L. I., Jie, Z., Minmin, Y. U., & Hui, Y. A. O. (2022). Sitagliptin phosphate tablet and preparation method thereof. CN114159401.
Seval, A., Muge, U. B., Fatih, S., Onur, M., Ezel, U., & Seda, A. (2022). A tablet formulation comprising sitagliptin and metformin. WO2022035400.
Qihui, X. I. E., Chaoyu, Y., Tiantian, N. I. E., & Longlong, W. (2022). Pharmaceutical composition containing sitagliptin and metformin, and preparation method thereof. CN114042051.
Yongjie, Z., & Ying, Z. (2022). Sitagliptin and metformin double-layer sustained release tablet and preparation method thereof. CN114010612.
Yuyuan, W. U., Hui, Z., Tiantian, X. I. E., & Maojia, Y. (2022). Analysis method for detecting release rate of sitagliptin metformin hydrochloride sustained release tablet. CN113945661.
Yusheng, P. A. N., Xinxin, X., Cenbo, C., Hao, L. I., & Haixiang, W. (2022). Compound sustained-release tablet of epalrestat and sitagliptin or pharmaceutically acceptable salt thereof and preparation method thereof. CN113925838A.
Sik, K. I. M. B. O., Wook, T. A. K. J. I. N., Hyun, C. H. O. J., Taek, I. M. H. O., & IL, K. I. M. Y. (2022). Composite formulation comprising sitagliptin and dapagliflozin and preparation method therefor. WO2022010078A1.
Xiaojie, W., Dingchao, Q. I., Baocheng, Z., Guokai, L. I. U., & Baoquan, Z. (2022). Preparation method of 3-hydroxy-1-adamantane methyl ketone and method for synthesizing saxagliptin. CN114621068.
Kenji, N., Wataru, I., Ayane, N., & Daiki, B. (2022). Saxagliptin-containing preparation and its manufacturing method. JP2022003016.
Aydan, O., Nur, P. A., & Fatih, S. (2022). A process for formulations of linagliptin or a pharmaceutically acceptable salt thereof. WO2022173406A1.
Takahisa, S., & Yukiko, K. (2022). Linagliptin-containing pharmaceutical composition with excellent thermal stability. JP2022097335.
Ali, T., Hasan, T. A. L. I., & Mehtap, S. (2022). Pharmaceutical formulations of linagliptin. EP4019003.
Meinicke, T., & Eynatten, M. V. O. N. (2022). Combination of linagliptin and metformin. JP2022093381.
Yasushi, F., & Katsuhiko, O. (2022). Linagliptin-containing orally disintegrating tablet. WO2022102457.
Takuma, T., & Masaya, F. (2022). Linagliptin-containing granule and pharmaceutical composition. JP2022074105A.
Georg, B., Julia, F. K., Venkata, V., & Tracy, W. (2022). Pharmaceutical composition, methods for treating and uses thereof. US20220105043A1.
Rentaro, I., Satoshi, K., & Junichi, K. (2022). Pharmaceutical formulation containing linagliptin and photostabilizing ingredient. JP2022012138A.
Sermet, B. S., Selin, K. U., Gulcin, T. O. K., & Fatih, S. (2022). Pharmaceutical compositions comprising alogliptin. WO2022146344A1.
Sermet, B. S., Selin, K. U., Bulent, D., Gulcin, T. O. K., & Fatih, S. (2022). Pharmaceutical capsule compositions of alogliptine. WO2022146355.
Gulcin, T. O. K., Ediz, Y., & Ali, T. (2022). A combination comprising alogliptin and metformin. EP3976014.
Qing, Z., Weibo, G. U. O., Lili, D., Jing, L. U. O., & Xiaoqin, L. I. U. (2022). Preparation method of alogliptin benzoate with high yield. CN114057685.
Acknowledgements
The authors would like to thank the Department of Pharmaceutics, MM College of Pharmacy, Maharishi Markandeshwar (Deemed to be University), Mullana-Ambala, Haryana, India, 133207 for providing facilities for the completion of this review.
Funding
None.
Author information
Authors and Affiliations
Contributions
Neha Tiwary (N.T.) and Neelam Sharma (N.S.) conceived this study and wrote the final manuscript; Sukhbir Singh (S.S.) and Tapan Behl (T.B.) revised and edited this manuscript; Ishrat Zahoor (I.Z.) prepared figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Research Involving Humans and Animals Statement
None.
Informed Consent
None.
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tiwary, N., Sharma, N., Singh, S. et al. Understanding the Pharmacological and Nanotechnological Facets of Dipeptidyl Peptidase-4 Inhibitors in Type II Diabetes Mellitus: a Paradigm in Therapeutics. BioNanoSci. 14, 211–229 (2024). https://doi.org/10.1007/s12668-023-01234-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01234-7