Abstract
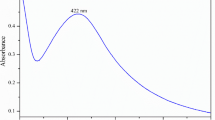
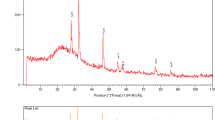
Biomolecules such as L-aspartic acid can be well-reducing and capping agents to produce metal nanoparticles with unique bioapplications. Silver nanoparticles (AgNPs) were synthesized using L-aspartic acid and evaluated as antibacterial and anticancer agents. The L-aspartic acid-AgNPs were characterized by utilizing spectroscopic instruments. Ultraviolet–visible (UV–Vis) spectroscopy gave peaks at 416 nm. Also, based on Fourier transform infrared spectroscopy (FTIR) analyses, it was determined that there are different functional groups of L-aspartic acid, namely, amino groups (-NH2), carboxylic acids (-COOH), and R-groups (side chains), that are responsible for bioreduction of Ag salt and the production of AgNPs. According to transmission electron microscopy (TEM), AgNPs are almost spherical in shape and have a nanoscale size of 11.3 nm. X-ray diffraction (XRD) study revealed a distinctive diffraction peak indicating crystalline nanoparticle formation, which agrees with AgNPs’ spherical structure. Field emission scanning electron microscopy (FE-SEM) was carried out with the energy-dispersive X-ray spectroscopy (EDX) analysis to determine the elemental composition of biosynthesized AgNPs, which included silver, oxygen, and carbon. Dynamic light scattering (DLS) was designated with a hydrodynamic radius of 109 nm, polydispersity (PdI) is good at 0.266, and a Z-potential of − 14.5 mV indicates good stability. AgNPs showed antibacterial activity against gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli). AgNPs have cytotoxicity 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay against breast cancer MCF-7 cell line, half-maximal inhibitory concentration (IC50) of 1159.6 µg/mL, and genotoxicity by comet assay, emphasizing apoptosis cells through cell cycle flow cytometry. Biogenic nano-formulations of L-aspartic acid-AgNPs possess antibacterial and anticancer therapeutic applications because they are safe, cost-effective, and scalable.















Similar content being viewed by others
Data Availability
The data that support the findings of this study are available within the article.
References
Mickymaray, S., Al Aboody, M. S., Eraqi, M. M., Alhoqail, W. A., Alothaim, A. S., & Suresh, K. (2023). Biopolymer chitosan surface engineering with magnesium oxide-pluronic-f127-escin nanoparticles on human breast carcinoma cell line and microbial strains. Nanomaterials, 1227, 2079–4991. https://doi.org/10.3390/nano13071227
Yusuf, M. (2019). Silver nanoparticles: Synthesis and applications. Handbook of Ecomaterials, 2343. https://doi.org/10.1007/978-3-319-68255-6_16.
Khosravimelal, S., Chizari, M., Farhadihosseinabadi, B., MoosazadehMoghaddam, M., & Gholipourmalekabadi, M. (2021). Fabrication and characterization of an antibacterial chitosan/silk fibroin electrospun nanofiber loaded with a cationic peptide for wound-dressing application. Journal of Materials Science: Materials in Medicine, 1–11, 0957–4530. https://doi.org/10.1007/s10856-021-06542-6
Yaqoob, A. A., Ahmad, H., Parveen, T., Ahmad, A., Oves, M., Ismail, I. M., Qari, H. A., Umar, K., & Mohamad Ibrahim, M. N. (2020). Recent advances in metal decorated nanomaterials and their various biological applications: A review. Frontiers in chemistry, 341, 2296–2646. https://doi.org/10.3389/fchem.2020.00341
Khandel, P., Shahi, S. K., Soni, D. K., Yadaw, R. K., & Kanwar, L. (2018). Alpinia calcarata: Potential source for the fabrication of bioactive silver nanoparticles. Nano convergence, 1–17, 2196–5404. https://doi.org/10.1186/s40580-018-0167-9
Moodley, J. S., Krishna, S. B. N., Pillay, K., & Govender, P. (2018). Green synthesis of silver nanoparticles from moringa oleifera leaf extracts and its antimicrobial potential. Advances in Natural Sciences: Nanoscience and Nanotechnology, 015011, 2043–6262. https://doi.org/10.1088/2043-6254/aaabb2
Awwad, A. M., Salem, N. M., & Abdeen, A. O. (2013). Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. International journal of Industrial chemistry, 1–6, 2228–5970. https://doi.org/10.1186/2228-5547-4-29
Rezazadeh, N. H., Buazar, F., & Matroodi, S. (2020). Synergistic effects of combinatorial chitosan and polyphenol biomolecules on enhanced antibacterial activity of biofunctionalized silver nanoparticles. Sci Rep, 1–13, 2045–2322. https://doi.org/10.1038/s41598-020-76726-7
Jeevanandam, J., Kiew, S. F., Boakye-Ansah, S., Lau, S. Y., Barhoum, A., Danquah, M. K., & Rodrigues, J. (2022). Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale, 2534-2571.https://doi.org/10.1039/D1NR08144F
Rafique, M., Sadaf, I., Rafique, M. S., & Tahir, M. B. (2017). A review on green synthesis of silver nanoparticles and their applications. Artificial cells, Nanomedicine, and Biotechnology, 1272–1291, 2169–1401. https://doi.org/10.1080/21691401.2016.1241792
Gardea-Torresdey, J. L., Gomez, E., Peralta-Videa, J. R., Parsons, J. G., Troiani, H., & Jose-Yacaman, M. (2003). Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles. Langmuir, 1357–1361, 0743–7463. https://doi.org/10.1021/la020835i
Jha, A. K., Prasad, K., & Kulkarni, A. (2009). Synthesis of tio2 nanoparticles using microorganisms. Colloids and surfaces B: Biointerfaces, 226–229, 0927–7765. https://doi.org/10.1016/j.colsurfb.2009.02.007
Shankar, S. S., Rai, A., Ahmad, A., & Sastry, M. (2004). Rapid synthesis of au, ag, and bimetallic au core–ag shell nanoparticles using neem (azadirachta indica) leaf broth. Journal of Colloid and Interface Science, 496–502, 0021–9797.
Hasan, S. (2015). A review on nanoparticles: Their synthesis and types. Research Journal of Recent Sciences, 2277, 2502.
Roy, M., Mukherjee, P., Mandal, B. P., Sharma, R. K., Tyagi A. K., & Kale S. P. (2012). Biomimetic synthesis of nanocrystalline silver sol using cysteine: Stability aspects and antibacterial activities. Rsc Advances, 6496–6503. https://doi.org/10.1039/C2RA00785A.
Qin, D., Yang, G., Wang, Y., Zhou, Y., & Zhang, L. (2019). Green synthesis of biocompatible trypsin-conjugated ag nanocomposite with antibacterial activity. Applied Surface Science, 528–536, 0169–4332. https://doi.org/10.1016/j.apsusc.2018.11.057
Selvakannan, P., Swami, A., Srisathiyanarayanan, D., Shirude, P. S., Pasricha, R., Mandale, A. B., & Sastry, M. (2004). Synthesis of aqueous au core− ag shell nanoparticles using tyrosine as a ph-dependent reducing agent and assembling phase-transferred silver nanoparticles at the air− water interface. Langmuir, 7825–7836, 0743–7463. https://doi.org/10.1021/la049258j
Wypij, M., Jędrzejewski, T., Trzcińska-Wencel, J., Ostrowski, M., Rai, M., & Golińska, P. (2021). Green synthesized silver nanoparticles: Antibacterial and anticancer activities, biocompatibility, and analyses of surface-attached proteins. Frontiers in microbiology, 632505, 1664–302X. https://doi.org/10.3389/fmicb.2021.632505
Bidan, A. K., & Al-Ali, Z. S. A. (2022). Biomedical evaluation of biosynthesized silver nanoparticles by jasminum sambac (l.) aiton against breast cancer cell line, and both bacterial strains colonies. International Journal of Nanoscience, 2250042, 0219-581X. https://doi.org/10.1142/S0219581X22500429
Shankar, S., & Rhim, J.-W. (2015). Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohydrate polymers, 353–363, 0144–8617. https://doi.org/10.1016/j.carbpol.2015.05.018
Adelnia, H., Tran, H. D., Little, P. J., Blakey, I., & Ta, H. T. (2021). Poly (aspartic acid) in biomedical applications: From polymerization, modification, properties, degradation, and biocompatibility to applications. ACS Biomaterials Science & Engineering, 2083–2105, 2373–9878. https://doi.org/10.1021/acsbiomaterials.1c00150
Ateeq, B., Farah, M. A., & Ahmad, W. (2005). Detection of DNA damage by alkaline single cell gel electrophoresis in 2, 4-dichlorophenoxyacetic-acid-and butachlor-exposed erythrocytes of clarias batrachus. Ecotoxicology and environmental safety, 348–354, 0147–6513. https://doi.org/10.1016/j.ecoenv.2004.12.011
Essghaier, B., Dridi, R., Mottola, F., Rocco, L., Zid, M. F., & Hannachi, H. (2023). Biosynthesis and characterization of silver nanoparticles from the extremophile plant aeonium haworthii and their antioxidant, antimicrobial and anti-diabetic capacities. Nanomaterials, 100, 2079–4991. https://doi.org/10.3390/nano13010100
Wasilewska, A., Klekotka, U., Zambrzycka, M., Zambrowski, G., Święcicka, I., & Kalska-Szostko, B. (2023). Physico-chemical properties and antimicrobial activity of silver nanoparticles fabricated by green synthesis. Food Chemistry, 133960, 0308–8146. https://doi.org/10.1016/j.foodchem.2022.133960
Ammari, H., Deng, Y., & Millien, P. (2016). Surface plasmon resonance of nanoparticles and applications in imaging. Archive for Rational Mechanics and Analysis, 109–153, 0003–9527. https://doi.org/10.1007/s00205-015-0928-0
Răcuciu, M., Barbu-Tudoran, L., Oancea, S., Drăghici, O., Morosanu, C., Grigoras, M., Brînză, F., & Creangă, D. E. (2022). Aspartic acid stabilized iron oxide nanoparticles for biomedical applications. Nanomaterials, 1151, 2079–4991. https://doi.org/10.3390/nano12071151
Bunaciu, A. A., Udriştioiu, E. G., & Aboul-Enein, H. Y. (2015). X-ray diffraction: Instrumentation and applications. Critical reviews in analytical chemistry, 289–299, 1040–8347. https://doi.org/10.1080/10408347.2014.949616
Agnihotri, S., Mukherji, S., & Mukherji, S. (2014). Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. Rsc Advances, 3974-3983https://doi.org/10.1039/C3RA44507K
Ali, I. A. M., Ahmed, A. B., & Al-Ahmed, H. I. (2023). Green synthesis and characterization of silver nanoparticles for reducing the damage to sperm parameters in diabetic compared to metformin. Sci Rep, 2256, 2045–2322. https://doi.org/10.1038/s41598-023-29412-3
Sadeghi, B., & Gholamhoseinpoor, F. (2015). A study on the stability and green synthesis of silver nanoparticles using ziziphora tenuior (zt) extract at room temperature. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 310–315, 1386–1425. https://doi.org/10.1016/j.saa.2014.06.046
Alharbi, N. S., Alsubhi, N. S., & Felimban, A. I. (2022). Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. Journal of Radiation Research and Applied Sciences, 109–124, 1687–8507. https://doi.org/10.1016/j.jrras.2022.06.012
Guo, Z., Tian, Y., Zhang, D., Wang, T., & Wu, M. (2019). A novel stick-slip based linear actuator using bi-directional motion of micropositioner. Mechanical Systems and Signal Processing, 37–49, 0888–3270. https://doi.org/10.1016/j.ymssp.2019.03.025
Bamal, D., Singh, A., Chaudhary, G., Kumar, M., Singh, M., Rani, N., Mundlia, P., & Sehrawat, A. R. (2021). Silver nanoparticles biosynthesis, characterization, antimicrobial activities, applications, cytotoxicity and safety issues: An updated review. Nanomaterials, 2086, 2079–4991. https://doi.org/10.3390/nano11082086
Harwood, C. R., Mouillon, J.-M., Pohl, S., & Arnau, J. (2018). Secondary metabolite production and the safety of industrially important members of the bacillus subtilis group. FEMS Microbiology Reviews, 721–738, 0168–6445. https://doi.org/10.1093/femsre/fuy028
Alzubaidi, A. K., Al-Kaabi, W. J., Ali, A. A., Albukhaty, S., Al-Karagoly, H., Sulaiman, G. M., Asiri, M., & Khane, Y. (2023). Green synthesis and characterization of silver nanoparticles using flaxseed extract and evaluation of their antibacterial and antioxidant activities. Applied Sciences, 2182, 2076–3417.
Khane, Y., Benouis, K., Albukhaty, S., Sulaiman, G. M., Abomughaid, M. M., Al, Ali A., Aouf, D., Fenniche, F., Khane, S., & Chaibi, W. (2022). Green synthesis of silver nanoparticles using aqueous citrus limon zest extract: Characterization and evaluation of their antioxidant and antimicrobial properties. Nanomaterials, 2013, 2079–4991. https://doi.org/10.3390/nano12122013
Nayak, S., Bhat, M. P., Udayashankar, A., Lakshmeesha, T., Geetha, N., & Jogaiah, S. (2020). Biosynthesis and characterization of dillenia indica-mediated silver nanoparticles and their biological activity. Applied Organometallic Chemistry, e5567, 0268–2605. https://doi.org/10.1002/aoc.5567
Balasubramanian, S., Kala, S. M. J., & Pushparaj, T. L. (2020). Biogenic synthesis of gold nanoparticles using jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. Journal of Drug Delivery Science and Technology, 101620, 1773–2247. https://doi.org/10.1016/j.jddst.2020.101620
Abd-Elhady, H. M., Ashor, M. A., Hazem, A., Saleh, F. M., Selim, S., El Nahhas, N., Abdel-Hafez, S. H., Sayed, S., & Hassan, E. A. (2022). Biosynthesis and characterization of extracellular silver nanoparticles from streptomyces aizuneusis: Antimicrobial, anti larval, and anticancer activities. Molecules, 212, 1420–3049. https://doi.org/10.3390/molecules27010212
Biswal, A. K., & Misra, P. K. (2020). Biosynthesis and characterization of silver nanoparticles for prospective application in food packaging and biomedical fields. Materials Chemistry and Physics, 123014, 0254–0584. https://doi.org/10.1016/j.matchemphys.2020.123014
Nayak, B., & Misra, P. K. (2019). Recognition of the surface characteristics and electrical properties of a nanocrystalline hydroxyapatite synthesized from pila globosa shells for versatile applications. Materials Chemistry and Physics, 187–196, 0254–0584. https://doi.org/10.1016/j.matchemphys.2019.03.068
Bruna, T., Maldonado-Bravo, F., Jara, P., & Caro, N. (2021). Silver nanoparticles and their antibacterial applications. International Journal of Molecular Sciences, 1422–0067. https://doi.org/10.3390/ijms22137202.
Yin, I. X., Zhang, J., Zhao, I. S., Mei, M. L., Li, Q., & Chu, C. H. (2020). The antibacterial mechanism of silver nanoparticles and its application in dentistry. International journal of nanomedicine, 2555–2562, 1178–2013. https://doi.org/10.2147/IJN.S246764
Tang, S., & Zheng, J. (2018). Antibacterial activity of silver nanoparticles: Structural effects. Advanced healthcare materials, 1701503, 2192–2640.
Swamy, P. S., Bhat, M., & Nayaka, S. (2022). Amycolatopsis sp. Strain mn235945 mediated biosynthesis of silver nanoparticles: Characterization, antimicrobial and anticancer activity against hela and mcf-7 cell lines. Indian Journal of Pharmaceutical Sciences, 84(5). https://doi.org/10.36468/pharmaceutical-sciences.1012
Shashiraj, K. N., Hugar, A., Kumar, R. S., Rudrappa, M., Bhat, M. P., Almansour, A. I., Perumal, K., & Nayaka, S. (2023). Exploring the antimicrobial, anticancer, and apoptosis inducing ability of biofabricated silver nanoparticles using lagerstroemia speciosa flower buds against the human osteosarcoma (mg-63) cell line via flow cytometry. Bioengineering, 821, 2306–5354. https://doi.org/10.3390/bioengineering10070821
Karimi, J., & Mohsenzadeh, S. (2015). Rapid, green, and eco-friendly biosynthesis of copper nanoparticles using flower extract of aloe vera.Synthesis and Reactivity in Inorganic. Metal-Organic, and Nano-Metal Chemistry, 895–898, 1553–3174.
Akhtar, S., Asiri, S., Khan, F. A., Gunday, S., Iqbal, A., Alrushaid, N., Labib, O., Deen, G., & Henari, F. (2022). Formulation of gold nanoparticles with hibiscus and curcumin extracts induced anti-cancer activity. Arabian Journal of Chemistry, 103594, 1878–5352. https://doi.org/10.1016/j.arabjc.2021.103594
Kalaiselvan, V., & Rajasekaran, A. (2009). Biosynthesis of silver nanoparticles from aspergillus niger and evaluation of its wound healing activity in experimental rat model. International Journal of PharmTech Research, 4, 1523–1529.
Asaduzzaman, M., & Yeasmin, T. (2006). Antineoplastic properties of phyto-synthesized silver nanoparticles from hibiscus sabdariffa linn. Bark extract. https://doi.org/10.1074/jbc.M605937200
Almofti, M. R., Ichikawa, T., Yamashita, K., Terada, H., & Shinohara, Y. (2003). Silver ion induces a cyclosporine a-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome c. Journal of Biochemistry, 43–49, 1756–2651. https://doi.org/10.1093/jb/mvg111
Foldbjerg, R., Dang, D. A., & Autrup, H. (2011). Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, a549. Archives of Toxicology, 743–750, 1432–0738. https://doi.org/10.1007/s00204-010-0545-5
Dos Santos, C. A., Seckler, M. M., Ingle, A. P., Gupta, I., Galdiero, S., Galdiero, M., Gade, A., & Rai, M. (2014). Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. Journal of pharmaceutical sciences, 1931–1944, 0022–3549. https://doi.org/10.1002/jps.24001
El-Naggar, N.E.-A., Hussein, M. H., & El-Sawah, A. A. (2018). Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Scientific Reports, 8925, 2045–2322. https://doi.org/10.1038/s41598-018-27276-6
Hamouda, R. A., Hussein, M. H., Abo-Elmagd, R. A., & Bawazir, S. S. (2019). Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium oscillatoria limnetica. Scientific Reports, 13071, 2045–2322. https://doi.org/10.1038/s41598-019-49444-y
Dey, S., Fageria, L., Sharma, A., Mukherjee, S., Pande, S., Chowdhury, R., & Chowdhury, S. (2022). Silver nanoparticle-induced alteration of mitochondrial and er homeostasis affects human breast cancer cell fate. Toxicology Reports, 1977–1984, 2214–7500. https://doi.org/10.1016/j.toxrep.2022.10.017
Ranjitham, A. M., Suja, R., Caroling, G., & Tiwari, S. (2013). In vitro evaluation of antioxidant, antimicrobial, anticancer activities and characterisation of brassica oleracea. Var. Bortrytis. L synthesized silver nanoparticles. International Journal of Pharmacy and Pharmaceutical Sciences, 5(4), 239–251.
El-Naggar, N.E.-A., Hussein, M. H., & El-Sawah, A. A. (2017). Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Scientific Reports, 10844, 2045–2322. https://doi.org/10.1038/s41598-017-11121-3
Sulaiman, G. M., Tawfeeq, A. T., & Naji, A. S. (2018). Biosynthesis, characterization of magnetic iron oxide nanoparticles and evaluations of the cytotoxicity and DNA damage of human breast carcinoma cell lines. Artificial Cells, Nanomedicine, and Biotechnology, 1215–1229, 2169–1401. https://doi.org/10.1080/21691401.2017.1366335
Chaturvedi, V. K., Yadav, N., Rai, N. K., Ellah, N. H. A., Bohara, R. A., Rehan, I. F., Marraiki, N., Batiha, G.E.-S., Hetta, H. F., & Singh, M. P. (2020). Pleurotus sajor-caju-mediated synthesis of silver and gold nanoparticles active against colon cancer cell lines: A new era of herbonanoceutics. Molecules, 3091, 1420–3049. https://doi.org/10.3390/molecules25133091
Oladipo, A. O., Unuofin, J. O., Lebelo, S. L., & Msagati, T. A. M. (2022). Phytochemical-stabilized platinum-decorated silver nanocubes inhibit adenocarcinoma cells and enhance antioxidant effects by promoting apoptosis via cell cycle arrest. Pharmaceutics, 2541, 1999–4923. https://doi.org/10.3390/pharmaceutics14112541
Mokhtar, F. A., Selim, N. M., Elhawary, S. S., Abd El Hadi, S. R., Hetta, M. H., Albalawi, M. A., Shati, A. A., Alfaifi, M. Y., Elbehairi, S. E. I., & Fahmy, L. I. (2022). Green biosynthesis of silver nanoparticles using annona glabra and annona squamosa extracts with antimicrobial, anticancer, apoptosis potentials, assisted by in silico modeling, and metabolic profiling. Pharmaceuticals, 1354, 1424–8247. https://doi.org/10.3390/ph15111354
Austin, L. A., Kang, B., Yen, C.-W., & El-Sayed, M. A. (2011). Nuclear targeted silver nanospheres perturb the cancer cell cycle differently than those of nanogold. Bioconjugate chemistry, 2324–2331, 1043–1802. https://doi.org/10.1021/bc200386m
Noorbazargan, H., Amintehrani, S., Dolatabadi, A., Mashayekhi, A., Khayam, N., Moulavi, P., Naghizadeh, M., Mirzaie, A., Mirzaei Rad, F., & Kavousi, M. (2021). Anti-cancer & anti-metastasis properties of bioorganic-capped silver nanoparticles fabricated from juniperus chinensis extract against lung cancer cells. AMB Express, 61, 2191–0855. https://doi.org/10.1186/s13568-021-01216-6
Das, S., Das, J., Samadder, A., Bhattacharyya, S. S., Das, D., & Khuda-Bukhsh, A. R. (2013). Biosynthesized silver nanoparticles by ethanolic extracts of phytolacca decandra, gelsemium sempervirens, hydrastis canadensis and thuja occidentalis induce differential cytotoxicity through g2/m arrest in a375 cells. Colloids and surfaces B: Biointerfaces, 325–336, 0927–7765.
Zhang, G., Zhu, Y., Wang, Y., Wei, D., Wu, Y., Zheng, L., Bai, H., Xiao, H., & Zhang, Z. (2019). Ph/redox sensitive nanoparticles with platinum (iv) prodrugs and doxorubicin enhance chemotherapy in ovarian cancer. Rsc Advances, 20513-20517.https://doi.org/10.1039/C9RA04034J.
Author information
Authors and Affiliations
Contributions
ZS conceived and designed the study. She contributed to the writing and interpreting of the results of the manuscript. SH synthesized and purified the AgNPs. She contributed to interpreting the results and writing the initial draft of the manuscript. AA characterized the AgNPs and contributed to interpreting the results. All authors participated in all manuscript requirements to produce a final version after being read and agreed upon by everyone.
Corresponding author
Ethics declarations
Ethical Approval
None.
Competing Interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-Majeed, S.H.A., Al-Ali, Z.S.A. & Turki, A.A. Biomedical Assessment of Silver Nanoparticles Derived from L-Aspartic Acid Against Breast Cancer Cell Lines and Bacteria Strains. BioNanoSci. 13, 1833–1848 (2023). https://doi.org/10.1007/s12668-023-01198-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-023-01198-8