Abstract
Cervical cancer is one of the most prevalent cancers among women. The currently available treatments like immunotherapy, radiotherapy, chemotherapy, and surgery have many side effects, including urinary bladder infections, gynecological morbidity, and anal sphincter dysfunction. The gut microbiome, Lactobacillus plantarum, and Lactobacillus rhamnosus have the ability to recognize normal cells as well as cancerous cells. Chitosan encapsulated nanoparticles enhance the targeted drug delivery without any side effects. The present investigation demonstrates the efficacy of chitosan encapsulated piperine and probiotic nanoparticles against cervical cancer cells. Chitosan encapsulated nanoparticles are prepared by the tripolyphosphate cross-linking method. The characterization study of nanoparticles was carried out through UV–visible spectroscopy, dynamic light scattering analysis, zeta potential analysis, and SEM imaging techniques. The cytotoxic effect of chitosan encapsulated piperine, Lactobacillus plantarum, and Lactobacillus rhamnosus nanoparticles on HeLa cells was assessed by MTT assay. From the findings, we concluded that chitosan encapsulated probiotic Lactobacillus rhamnosus nanoparticles showed potent anti-cancer efficacy against HeLa cell lines.
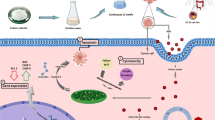
Graphical abstract






Similar content being viewed by others
Availability of Data and Materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Code Availability
Not applicable.
Abbreviations
- HPV:
-
Human papillomavirus
- L. plantarum :
-
Lactobacillus plantarum
- L. rhamnosus :
-
Lactobacillus rhamnosus
- PTEN:
-
Phosphatase and tensin homolog
- MAPK:
-
Mitogen-activated protein kinases
- DLS:
-
Dynamic light scattering
- TPP:
-
Sodium tripolyphosphate
- PBS:
-
Phosphate-buffered saline
- NaCl:
-
Sodium chloride
- FBS:
-
Fetal bovine serum
- MTT:
-
(3-(4,5-Dimethyl thiazolyl-2)-2, 5-diphenyltetrazolium bromide)
- DMSO:
-
Dimethyl sulfoxide
- SEM:
-
Scanning electron microscopy
- CS-NPs:
-
Chitosan nanoparticles
- CS-PNPs:
-
Chitosan encapsulated piperine nanoparticles
- CS-LPNPs:
-
Chitosan encapsulated Lactobacillus plantarum nanoparticles
- CS-LRNPs:
-
Chitosan encapsulated Lactobacillus rhamnosus nanoparticles
- PDI:
-
Poly dispersity index
References
Small, W., et al. (Jul. 2017). Cervical cancer: A global health crisis. Cancer, 123(13), 2404–2412. https://doi.org/10.1002/cncr.30667
Kessler, T. A. (May 2017). Cervical cancer: Prevention and early detection. Seminars in Oncology Nursing, 33(2), 172–183. https://doi.org/10.1016/j.soncn.2017.02.005
García Arteaga, J. D. and Kybic, J. (2007) , “Automatic landmark detection for cervical image registration validation,” p. 65142S, https://doi.org/10.1117/12.708893
Pedersen, D., Bentzen, S. M., & Overgaard, J. (Jul. 1994). Early and late radiotherapeutic morbidity in 442 consecutive patients with locally advanced carcinoma of the uterine cervix. Int. J. Radiat. Oncol., 29(5), 941–952. https://doi.org/10.1016/0360-3016(94)90387-5
Sahoo, S. K., Parveen, S., & Panda, J. J. (Mar. 2007). “The present and future of nanotechnology in human health care”, Nanomedicine Nanotechnology. Biologie et Médecine, 3(1), 20–31. https://doi.org/10.1016/j.nano.2006.11.008
Zhang, L., Gu, F., Chan, J., Wang, A., Langer, R., & Farokhzad, O. (May 2008). Nanoparticles in medicine: Therapeutic applications and developments. Clinical Pharmacology and Therapeutics, 83(5), 761–769. https://doi.org/10.1038/sj.clpt.6100400
Zhang, M., et al. (Dec. 2016). A hyaluronidase-responsive nanoparticle-based drug delivery system for targeting colon cancer cells. Cancer Research, 76(24), 7208–7218. https://doi.org/10.1158/0008-5472.CAN-16-1681
Ajnai, G., Chiu, A., Kan, T., Cheng, C.-C., Tsai, T.-H., & Chang, J. (Dec. 2014). Trends of gold nanoparticle-based drug delivery system in cancer therapy. J. Exp. Clin. Med., 6(6), 172–178. https://doi.org/10.1016/j.jecm.2014.10.015
Srivastava, V., Gusain, D., & Sharma, Y. C. (Jun. 2015). Critical review on the toxicity of some widely used engineered nanoparticles. Industrial and Engineering Chemistry Research, 54(24), 6209–6233. https://doi.org/10.1021/acs.iecr.5b01610
P. Guiot and P. Couvreur, Polymeric nanoparticles and microspheres. CRC Press, 2018
Islam, S., Bhuiyan, M. A. R., & Islam, M. N. (Sep. 2017). Chitin and chitosan: Structure, properties and applications in biomedical engineering. Journal of Polymers and the Environment, 25(3), 854–866. https://doi.org/10.1007/s10924-016-0865-5
Saikia, C. and Gogoi, P. (2015). “Chitosan: A promising biopolymer in drug delivery applications,” J. Mol. Genet. Med., vol. s4, https://doi.org/10.4172/1747-0862.S4-006.
Yuan, Q., Hein, S., & Misra, R. D. K. (Jul. 2010). New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: Synthesis, characterization and in vitro drug delivery response. Acta Biomaterialia, 6(7), 2732–2739. https://doi.org/10.1016/j.actbio.2010.01.025
Li, J., et al. (Oct. 2018). Chitosan-based nanomaterials for drug delivery. Molecules, 23(10), 2661. https://doi.org/10.3390/molecules23102661
Zeng, Z. (2011). “Recent advances of chitosan nanoparticles as drug carriers,” Int. J. Nanomedicine, p. 765, https://doi.org/10.2147/IJN.S17296.
Agnihotri, S. A., Mallikarjuna, N. N., & Aminabhavi, T. M. (Nov. 2004). Recent advances on chitosan-based micro- and nanoparticles in drug delivery. Journal of Controlled Release, 100(1), 5–28. https://doi.org/10.1016/j.jconrel.2004.08.010
Hassani, S., Laouini, A., Fessi, H., & Charcosset, C. (Oct. 2015). Preparation of chitosan–TPP nanoparticles using microengineered membranes – Effect of parameters and encapsulation of tacrine. Colloids Surfaces A Physicochem. Eng. Asp., 482, 34–43. https://doi.org/10.1016/j.colsurfa.2015.04.006
Umadevi, P., Deepti, K., & Venugopal, D. V. R. (Nov. 2013). Synthesis, anticancer and antibacterial activities of piperine analogs. Medicinal Chemistry Research, 22(11), 5466–5471. https://doi.org/10.1007/s00044-013-0541-4
Dahiya, S., Rani, R., Dhingra, D., Kumar, S., & Dilbaghi, N. (Aug. 2018). Conjugation of epigallocatechin gallate and piperine into a zein nanocarrier: Implication on antioxidant and anticancer potential. Adv. Nat. Sci. Nanosci. Nanotechnol., 9(3), 035011. https://doi.org/10.1088/2043-6254/aad5c1
Lai, L., et al. (Apr. 2012). Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacologica Sinica, 33(4), 523–530. https://doi.org/10.1038/aps.2011.209
Katiyar, S. S., Muntimadugu, E., Rafeeqi, T. A., Domb, A. J., & Khan, W. (Sep. 2016). Co-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Delivery, 23(7), 2608–2616. https://doi.org/10.3109/10717544.2015.1039667
Jain, S., Meka, S. R. K., & Chatterjee, K. (Aug. 2016). Engineering a piperine eluting nanofibrous patch for cancer treatment. ACS Biomaterials Science & Engineering, 2(8), 1376–1385. https://doi.org/10.1021/acsbiomaterials.6b00297
Han, S., Liu, H., Yang, L., Cui, L., & Xu, Y. (Dec. 2017). Piperine (PP) enhanced mitomycin-C (MMC) therapy of human cervical cancer through suppressing Bcl-2 signaling pathway via inactivating STAT3/NF-κB. Biomedicine & Pharmacotherapy, 96, 1403–1410. https://doi.org/10.1016/j.biopha.2017.11.022
Baspinar, Y., Üstündas, M., Bayraktar, O., & Sezgin, C. (Mar. 2018). Curcumin and piperine loaded zein-chitosan nanoparticles: Development and in-vitro characterisation. Saudi Pharm. J., 26(3), 323–334. https://doi.org/10.1016/j.jsps.2018.01.010
Kumar, S. S. D., Surianarayanan, M., Vijayaraghavan, R., Mandal, A. B., & MacFarlane, D. R. (Jan. 2014). Curcumin loaded poly(2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid – In vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. European Journal of Pharmaceutical Sciences, 51, 34–44. https://doi.org/10.1016/j.ejps.2013.08.036
Elnaggar, Y. S. R., Etman, S. M., Abdelmonsif, D. A., & Abdallah, O. Y. (Oct. 2015). Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: Optimization, biological efficacy, and potential toxicity. Journal of Pharmaceutical Sciences, 104(10), 3544–3556. https://doi.org/10.1002/jps.24557
Walter, J. (Aug. 2008). Ecological role of lactobacilli in the gastrointestinal tract: Implications for fundamental and biomedical research. Applied and Environment Microbiology, 74(16), 4985–4996. https://doi.org/10.1128/AEM.00753-08
Reid, G., Jass, J., Sebulsky, M. T., & McCormick, J. K. (Oct. 2003). Potential uses of probiotics in clinical practice. Clinical Microbiology Reviews, 16(4), 658–672. https://doi.org/10.1128/CMR.16.4.658-672.2003
Makarova, K., et al. (Oct. 2006). Comparative genomics of the lactic acid bacteria. Proceedings of the National Academy of Sciences, 103(42), 15611–15616. https://doi.org/10.1073/pnas.0607117103
Fijan, S. (May 2014). Microorganisms with claimed probiotic properties: An overview of recent literature. International Journal of Environmental Research and Public Health, 11(5), 4745–4767. https://doi.org/10.3390/ijerph110504745
Paolillo, R., Romano Carratelli, C., Sorrentino, S., Mazzola, N., and Rizzo, A. (2009). “Immunomodulatory effects of Lactobacillus plantarum on human colon cancer cells,” Int. Immunopharmacol., vol. 9, no. 11, pp. 1265–1271, https://doi.org/10.1016/j.intimp.2009.07.008.
Fredriksen, L., Mathiesen, G., Sioud, M., & Eijsink, V. G. H. (Nov. 2010). Cell wall anchoring of the 37-kilodalton oncofetal antigen by Lactobacillus plantarum for mucosal cancer vaccine delivery. Applied and Environment Microbiology, 76(21), 7359–7362. https://doi.org/10.1128/AEM.01031-10
Sentürk, M., Ercan, F., & Yalcin, S. (Jan. 2020). The secondary metabolites produced by Lactobacillus plantarum downregulate BCL-2 and BUFFY genes on breast cancer cell line and model organism Drosophila melanogaster: Molecular docking approach. Cancer Chemotherapy and Pharmacology, 85(1), 33–45. https://doi.org/10.1007/s00280-019-03978-0
Luang-In, V., et al. (Aug. 2020). Cytotoxicity of Lactobacillus plantarum KK518 isolated from Pak-Sian Dong (Thai Fermented Gynandropsis pentaphylla DC.) against HepG2, MCF-7 and HeLa cancer cells. Pharmacogn. J., 12(5), 1050–1057. https://doi.org/10.5530/pj.2020.12.148
Rafter, J. (Sep. 2002). Lactic acid bacteria and cancer: Mechanistic perspective. British Journal of Nutrition, 88(S1), S89–S94. https://doi.org/10.1079/BJN2002633
Zhong, L. (2014). Emerging roles of lactic acid bacteria in protection against colorectal cancer. World Journal of Gastroenterology, 20(24), 7878. https://doi.org/10.3748/wjg.v20.i24.7878
Christensen, H. R., Frøkiær, H., & Pestka, J. J. (Jan. 2002). Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. The Journal of Immunology, 168(1), 171–178. https://doi.org/10.4049/jimmunol.168.1.171
Asoudeh-Fard, A., Barzegari, A., Dehnad, A., Bastani, S., Golchin, A., & Omidi, Y. (Jun. 2017). Lactobacillus plantarum induces apoptosis in oral cancer KB cells through upregulation of PTEN and downregulation of MAPK signalling pathways. BioImpacts: BI, 7(3), 193–198. https://doi.org/10.15171/bi.2017.22
Hu, J., et al. (Jun. 2015). Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumour-bearing mice. Journal of Biosciences, 40(2), 269–279. https://doi.org/10.1007/s12038-015-9518-4
Sharma, M., Chandel, D., & Shukla, G. (Jan. 2020). Antigenotoxicity and cytotoxic potentials of metabiotics extracted from isolated probiotic, Lactobacillus rhamnosus MD 14 on Caco-2 and HT-29 human colon cancer cells. Nutrition and Cancer, 72(1), 110–119. https://doi.org/10.1080/01635581.2019.1615514
Cook, M. T., Tzortzis, G., Charalampopoulos, D., & Khutoryanskiy, V. V. (Aug. 2012). Microencapsulation of probiotics for gastrointestinal delivery. Journal of Controlled Release, 162(1), 56–67. https://doi.org/10.1016/j.jconrel.2012.06.003
Călinoiu, L.-F., Ştefănescu, B., Pop, I., Muntean, L., & Vodnar, D. (Mar. 2019). Chitosan coating applications in probiotic microencapsulation. Coatings, 9(3), 194. https://doi.org/10.3390/coatings9030194
Vodnar, D. C., & Socaciu, C. (Jun. 2014). Selenium enriched green tea increase stability of Lactobacillus casei and Lactobacillus plantarum in chitosan coated alginate microcapsules during exposure to simulated gastrointestinal and refrigerated conditions. LWT - Food Sci. Technol., 57(1), 406–411. https://doi.org/10.1016/j.lwt.2013.12.043
Chávarri, M., Marañón, I., Ares, R., Ibáñez, F. C., Marzo, F., & Villarán, M. del C. (2010). Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol., 142(1–2), 185–189. https://doi.org/10.1016/j.ijfoodmicro.2010.06.022
Seow, S. W., Rahmat, J. N. B., Mohamed, A. A. K., Mahendran, R., Lee, Y. K., & Bay, B. H. (Nov. 2002). Lactobacillus species is more cytotoxic to human bladder cancer cells than Mycobacterium bovis (Bacillus Calmette-Guerin). Journal of Urology, 168(5), 2236–2239. https://doi.org/10.1016/S0022-5347(05)64362-5
Desai, K. G. (2016). Chitosan nanoparticles prepared by ionotropic gelation: An overview of recent advances. Crit. Rev. Ther. Drug Carr. Syst., 33(2), 107–158. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2016014850
Ebrahimnezhad, K. S.P., Khavarpour M. (2017) “Survival of Lactobacillus acidophilus as probiotic bacteria using chitosan nanoparticles,” Int. J. Eng., 30(4) https://doi.org/10.5829/idosi.ije.2017.30.04a.01
Rashedi, J., et al. (Aug. 2019). Anti-tumor effect of quercetin loaded chitosan nanoparticles on induced colon cancer in Wistar rats. Adv. Pharm. Bull., 9(3), 409–415. https://doi.org/10.15171/apb.2019.048
Kecel-Gunduz, S., et al. (Jun. 2020). In Silico design of AVP (4–5) peptide and synthesis, characterization and in vitro activity of chitosan nanoparticles. DARU J. Pharm. Sci., 28(1), 139–157. https://doi.org/10.1007/s40199-019-00325-9
D. S. Vijayalakshmi, V., Kousar, P. H. (2020). Optimization and characterization of chitosan based nanocarrier for the application of cancer drug delivery,” J. Crit. Rev., 7(07), https://doi.org/10.31838/jcr.07.07.139.
Ghadi, A., Mahjoub, S., Tabandeh, F., Talebnia, F., (2014) “Synthesis and optimization of chitosan nanoparticles: Potential applications in nanomedicine and biomedical engineering.,” Casp. J. Intern. Med., vol. 5, no. 3, pp. 156–61, 2014, [Online]. Available: http://www.ncbi.nlm.nih.gov/pubmed/25202443.
De Leersnyder, I., De Gelder, L., Van Driessche, I., & Vermeir, P. (Nov. 2019). Revealing the Importance of aging, environment, size and stabilization mechanisms on the stability of metal nanoparticles: A case study for silver nanoparticles in a minimally defined and complex undefined bacterial growth medium. Nanomaterials, 9(12), 1684. https://doi.org/10.3390/nano9121684
Ko, J., Park, H., Hwang, S., Park, J., & Lee, J. (Dec. 2002). Preparation and characterization of chitosan microparticles intended for controlled drug delivery. International Journal of Pharmaceutics, 249(1–2), 165–174. https://doi.org/10.1016/S0378-5173(02)00487-8
Gan, Q., Wang, T., Cochrane, C., & McCarron, P. (Aug. 2005). Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surfaces B Biointerfaces, 44(2–3), 65–73. https://doi.org/10.1016/j.colsurfb.2005.06.001
Sharifi, F., et al. (Oct. 2019). Zeta potential changing self-emulsifying drug delivery systems utilizing a novel Janus-headed surfactant: A promising strategy for enhanced mucus permeation. Journal of Molecular Liquids, 291, 111285. https://doi.org/10.1016/j.molliq.2019.111285
Shaikh, J., Ankola, D. D., Beniwal, V., Singh, D., & Kumar, M. N. V. R. (Jun. 2009). Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. European Journal of Pharmaceutical Sciences, 37(3–4), 223–230. https://doi.org/10.1016/j.ejps.2009.02.019
Tığlı Aydın, R. S., & Pulat, M. (2012). fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. Journal Nanomaterials., 2012, 1–10. https://doi.org/10.1155/2012/313961
Lim, E.-K., Huh, Y.-M., Yang, J., Lee, K., Suh, J.-S., & Haam, S. (Jun. 2011). pH-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by MRI. Advanced Materials, 23(21), 2436–2442. https://doi.org/10.1002/adma.201100351
Ahmad, N., et al. (Oct. 2016). Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of cerebral ischemia. International Journal of Biological Macromolecules, 91, 640–655. https://doi.org/10.1016/j.ijbiomac.2016.06.001
Ghaz-Jahanian, M. A., Abbaspour-Aghdam, F., Anarjan, N., Berenjian, A., & Jafarizadeh-Malmiri, H. (Mar. 2015). Application of chitosan-based nanocarriers in tumor-targeted drug delivery. Molecular Biotechnology, 57(3), 201–218. https://doi.org/10.1007/s12033-014-9816-3
Bahuguna, A., Khan, I., Bajpai, V. K., & Kang, S. C. (Apr. 2017). MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol., 12(2), 8. https://doi.org/10.3329/bjp.v12i2.30892
Acknowledgements
I and my co-authors would like to express our sincere gratitude towards Dr. K.M. Saradhadevi, Assistant Professor of the Department of Biochemistry, for her constant moral support and guidance throughout this research work.
Author information
Authors and Affiliations
Contributions
Gayathiri Gunasangkaran: Drafting the article and submitting the final version of the article.
Anjali K. Ravi: Drafting the article and submitting the final version of the article.
Vijaya Anand Arumugam: Co- author who might assist the corresponding author and first author in writing the article.
Saradhadevi Muthukrishnan: Corresponding author make sustainable contribution for the intellectual input and designing the whole paper.
Corresponding author
Ethics declarations
Research Involving Human Participants and Their Data or Biological Material
None.
Research Involving Animals and Their Data or Biological Material
None.
Utilization of Plants, Algae, and Fungi
Not applicable.
Ethics Approval and Consent to Participate
None.
Consent for Publication
The consent of all the authors has taken to publish the research in this journal.
Conflict of Interest
The authors declare no competing interests.
Funding
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gunasangkaran, G., Ravi, A.K., Arumugam, V.A. et al. Preparation, Characterization, and Anticancer Efficacy of Chitosan, Chitosan Encapsulated Piperine and Probiotics (Lactobacillus plantarum (MTCC-1407), and Lactobacillus rhamnosus (MTCC-1423) Nanoparticles. BioNanoSci. 12, 527–539 (2022). https://doi.org/10.1007/s12668-022-00961-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-022-00961-7