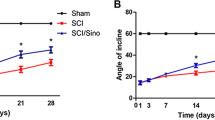
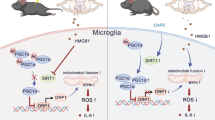
Abstract
One of the most important strategies for the treatment of spinal cord injury is searching for new and effective pharmacological neuroprotectors and regeneration stimulators. The derivatives of pyrimidine are universal stimulators of the regeneration of various tissues as they support the recovery of nervous structures. The protective effect of the cocrystal of 1,2-dihydro-4,6-dimethyl-1-(2-hydroxyethyl)-pyrimidinone-2 with para-aminobenzoic acid (compound conjugate III, CCIII) was explored on a rat model with a contusion spinal cord injury. Injection of CCIII significantly reduced the expression of tumor necrosis factor α (TNF-α), inhibited the synthesis of myeloperoxidase (MPO), matrix metalloproteinase 9 (MPP9), cyclooxygenase-2 (COX2), and macrophage marker CD68, and increased the level of superoxide dismutase 1 (SOD1). Additionally, the expression of caspase-7 markers in the damaged tissue decreased under the action of CCIII. Treatment with the CCIII maintained a population of Olig2-positive myelin-forming cells at 30 days post-injury. The detected therapeutic effect is comparable with that of riluzole.






Similar content being viewed by others
References
Bar-Or, A., Nuttall, R. K., Duddy, M., Alter, A., Kim, H. J., Ifergan, I., Pennington, C. J., Bourgoin, P., Edwards, D. R., & Yong, V. W. (2003). Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain, 126, 2738–2749. https://doi.org/10.1093/brain/awg285.
Casella, G. T., Bunge, M. B., & Wood, P. M. (2004). Improved immunocytochemical identification of neural, endothelial, and inflammatory cell types in paraffin-embedded injured adult rat spinal cord. Journal of Neuroscience Methods, 139, 1–11. https://doi.org/10.1016/j.jneumeth.2004.04.008.
Cheah, B. C., Vucic, S., Krishnan, A. V., & Kiernan, M. C. (2010). Riluzole, neuroprotection and amyotrophic lateral sclerosis. Current Medicinal Chemistry, 17, 1942–1199. https://doi.org/10.2174/092986710791163939.
Chelyshev, Y. A., & Raginov, I. S. (2002). Effect of stimulation of nerve regeneration on posttraumatic neuronal survival in dorsal root ganglia. Bulletin of Experimental Biology and Medicine, 134, 597–599. https://doi.org/10.1023/A:1022929632535.
Dubreuil, C. I., Winton, M. J., & McKerracher, L. (2003). Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. The Journal of Cell Biology, 162, 233–243. https://doi.org/10.1083/jcb.200301080.
Erschbamer, M. K., Hofstetter, C. P., & Olson, L. (2005). RhoA, RhoB, RhoC, Rac1, Cdc42, and Tc10 mRNA levels in spinal cord, sensory ganglia, and corticospinal tract neurons and long-lasting specific changes following spinal cord injury. The Journal of Comparative Neurology, 484, 224–233. https://doi.org/10.1002/cne.20471.
Fehlings, M. G., Nakashima, H., Nagoshi, N., Chow, D. S., Grossman, R. G., & Kopjar, B. (2016). Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord, 54, 8–15. https://doi.org/10.1038/sc.2015.95.
Figiel, I. (2008). Pro-inflammatory cytokine TNF-alpha as a neuroprotective agent in the brain. Acta Neurobiologiae Experimentalis (Wars), 68, 526–534.
Fleming, J. C., Norenberg, M. D., Ramsay, D. A., Dekaban, G. A., Marcillo, A. E., Saenz, A. D., Pasquale-Styles, M., Dietrich, W. D., & Weaver, L. C. (2006). The cellular inflammatory response in human spinal cords after injury. Brain, 129, 3249–3269. https://doi.org/10.1093/brain/awl296.
Fontaine, V., Mohand-Said, S., Hanoteau, N., Fuchs, C., Pfizenmaier, K., & Eisel, U. (2002). Neurodegenerative and neuroprotective effects of tumor necrosis factor (TNF) in retinal ischemia: opposite roles of TNF receptor 1 and TNF receptor 2. The Journal of Neuroscience, 22, RC216. https://doi.org/10.1523/JNEUROSCI.22-07-j0001.2002.
Grossman, R. G., Fehlings, M. G., Frankowski, R. F., Burau, K. D., Chow, D. S., Tator, C., Teng, A., Toups, E. G., Harrop, J. S., Aarabi, B., Shaffrey, C. I., Johnson, M. M., Harkema, S. J., Boakye, M., Guest, J. D., & Wilson, J. R. (2014). A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. Journal of Neurotrauma, 31, 239–255. https://doi.org/10.1089/neu.2013.2969.
Kopp, M. A., Liebscher, T., Watzlawick, R., Martus, P., Laufer, S., Blex, C., Schindler, R., Jungehulsing, G. J., Knüppel, S., Kreutzträger, M., Ekkernkamp, A., Dirnagl, U., Strittmatter, S. M., Niedeggen, A., & Schwab, J. M. (2016). SCISSOR-Spinal Cord Injury Study on Small molecule-derived Rho inhibition: a clinical study protocol. BMJ Open, 6, e010651. https://doi.org/10.1136/bmjopen-2015-010651.
Masgutov, R., Raginov, I., Fomina, G., Kozlova, M., & Chelyshev, Y. (2006). Stimulation of the rat’s sciatic nerve regeneration by local treatment with Xymedon. Cellular and Molecular Neurobiology, 26, 1413–1421. https://doi.org/10.1007/s10571-006-9055-7.
Mukhamedshina, Y. O., Garanina, E. E., Masgutova, G. A., Galieva, L. R., Sanatova, E. R., Chelyshev, Y. A., & Rizvanov, A. A. (2016). Assessment of glial scar, tissue sparing, behavioral recovery and axonal regeneration following acute transplantation of genetically modified human umbilical cord blood cells in a rat model of spinal cord contusion. PLoS One, 11, e0151745. https://doi.org/10.1371/journal.pone.0151745.
Nakazawa, T., Kayama, M., Ryu, M., Kunikata, H., Watanabe, R., Yasuda, M., Kinugawa, J., Vavvas, D., & Miller, J. W. (2011). Tumor necrosis factor-alpha mediates photoreceptor death in a rodent model of retinal detachment. Investigative Ophthalmology & Visual Science, 52, 1384–1391. https://doi.org/10.1167/iovs.10-6509.
Pedraza CE, Taylor C, Pereira A, Seng M, Tham CS, Izrael M, Webb M, (2014) Induction of oligodendrocyte differentiation and in vitro myelination by inhibition of rho-associated kinase. ASN Neuro, 6. https://doi.org/10.1177/1759091414538134.
Pekny, M., Wilhelmsson, U., & Pekna, M. (2014). The dual role of astrocyte activation and reactive gliosis. Neuroscience Letters, 565, 30–38. https://doi.org/10.1016/j.neulet.2013.12.071.
Povysheva, T., Shmarov, M., Logunov, D., Naroditsky, B., Shulman, I., Ogurcov, S., Kolesnikov, P., Islamov, R., & Chelyshev, Y. (2017). Post-spinal cord injury astrocyte-mediated functional recovery in rats after intraspinal injection of the recombinant adenoviral vectors Ad5-VEGF and Ad5-ANG. Journal of Neurosurgery. Spine, 27, 105–115. https://doi.org/10.3171/2016.9.SPINE15959.
Povysheva, T. V., Semenov, V. E., Galyametdinova, I. V., Reznik, V. S., Knni, K. S., Kolesnikov, P. E., & Chelyshev, Y. A. (2016). New Xymedon analogues for stimulation of posttraumatic regeneration of the spinal cord in rats. Bulletin of Experimental Biology and Medicine, 162, 220–224. https://doi.org/10.1007/s10517-016-3580-2.
Raginov, I. S., Chelyshev, Y. A., & Shagidullin, T. F. (2004). Interaction of sensory neurons and satellite cells during stimulation of nerve regeneration. Neuroscience and Behavioral Physiology, 34, 79–81. https://doi.org/10.1023/B:NEAB.0000003250.44648.5c.
Satkunendrarajah, K., Nassiri, F., Karadimas, S. K., Lip, A., Yao, G., & Fehlings, M. G. (2016). Riluzole promotes motor and respiratory recovery associated with enhanced neuronal survival and function following high cervical spinal hemisection. Experimental Neurology, 276, 59–71. https://doi.org/10.1016/j.expneurol.2015.09.011.
Sofroniew, M. V. (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends in Neurosciences, 32, 638–647. https://doi.org/10.1016/j.tins.2009.08.002.
Takeuchi, H., Jin, S., Wang, J., Zhang, G., Kawanokuchi, J., Kuno, R., Sonobe, Y., Mizuno, T., & Suzumura, A. (2006). Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. The Journal of Biological Chemistry, 281, 21362–21368. https://doi.org/10.1074/jbc.M600504200.
Venters, H. D., Dantzer, R., & Kelley, K. W. (2000). Tumor necrosis factor-alpha induces neuronal death by silencing survival signals generated by the type I insulin-like growth factor receptor. Annals of the New York Academy of Sciences, 917, 210–220. https://doi.org/10.1111/j.1749-6632.2000.tb05385.x.
Viviani, B., Corsini, E., Galli, C. L., & Marinovich, M. (1998). Glia increase degeneration of hippocampal neurons through release of tumor necrosis factor-alpha. Toxicology and Applied Pharmacology, 150, 271–276. https://doi.org/10.1006/taap.1998.8406.
Vyshtakalyuk, A. B., Nazarov, N. G., Zobov, V. V., Abdulkhakov, S. R., Minnekhanova, O. A., Semenov, V. E., Galyametdinova, I. V., Cherepnev, G. V., & Reznik, V. S. (2017). Evaluation of the hepatoprotective effect of L-ascorbate 1-(2-hydroxyethyl)-4,6-dimethyl-1,2-dihydropyrimidine-2-one upon exposure to carbon tetrachloride. Bulletin of Experimental Biology and Medicine, 162, 340–342. https://doi.org/10.1007/s10517-017-3610-8.
Vyshtakalyuk, A. B., Nazarov, N. G., Zueva, I. V., Lantsova, A. V., Minnekhanova, O. A., Busygin, D. V., Porfiryev, A. G., Evtyugin, V. G., Reznik, V. S., & Zobov, V. V. (2013). Study of hepatoprotective effects of xymedon. Bulletin of Experimental Biology and Medicine, 155, 643–646. https://doi.org/10.1007/s10517-013-2215-0.
Wu, X., & Xu, X. M. (2016). RhoA/Rho kinase in spinal cord injury. Neural Regeneration Research, 11, 23–27. https://doi.org/10.4103/1673-5374.169601.
Wu, Y., Satkunendrarajah, K., & Fehlings, M. G. (2014). Riluzole improves outcome following ischemia-reperfusion injury to the spinal cord by preventing delayed paraplegia. Neuroscience, 265, 302–312. https://doi.org/10.1016/j.neuroscience.2014.01.059.
Wu, Y., Satkunendrarajah, K., Teng, Y., Chow, D. S., Buttigieg, J., & Fehlings, M. G. (2013). Delayed post-injury administration of riluzole is neuroprotective in a preclinical rodent model of cervical spinal cord injury. Journal of Neurotrauma, 30, 441–452. https://doi.org/10.1089/neu.2012.2622.
Acknowledgements
We would like to thank D. N. Fattakhova, Kazan State Medical University (Kazan, Russia) for their assistance in some experiments.
Funding
This work was funded by grant №14-50-00014 from the Russian Science Foundation and supported by Program of Competitive Growth of KFU.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Povysheva, T.V., Sabirova, S.R., Shashin, M.S. et al. Pyrimidine Derivative Ameliorates Spinal Cord Injury via Anti-apoptotic, Anti-inflammatory, and Antioxidant Effects and by Regulating Rho GTPases. BioNanoSci. 9, 224–234 (2019). https://doi.org/10.1007/s12668-018-0570-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-018-0570-z