Abstract
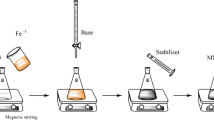
Sm3+-doped magnetic nanoparticles (NPs) were prepared via microwave-assisted polyol synthesis in ethylene glycol, poly(ethylene glycol) and mixed ethylene glycol—poly(ethylene glycol) solutions. In present work, the effects of organic solvent composition on particle size, particle size distribution, extent of agglomeration, and samarium content in prepared NPs were studied. The synthesized NPs were characterized by several techniques as follows: X-ray diffraction (XRD), transmission electron microscopy (TEM), thermogravimetry (TGA) and X-Ray fluorescence (XRF) analysis. XRD and TEM results showed formation of ~6.0–17.9 nm NPs having different microstructure characteristics (average particle size, particle size distribution, and agglomeration). The TGA analysis indicated the presence of organic components on the surface of NPs. Cytotoxic activity of the prepared magnetic NPs towards HeLa cells was evaluated by using standard live/dead assay in comparison to a control solution. It was shown that prepared magnetic NPs are characterized by low toxicity that makes possible their use for biomedical applications.





Similar content being viewed by others
References
Escamilla-Rivera, V., Uribe-Ramírez, M., González-Pozos, S., et al. (2016). Protein corona acts as a protective shield against Fe3O4-PEG inflammation and ROS-induced toxicity in human macrophages. Toxicol Lett, 240, 172–184.
Mody, V. V., Cox, A., Shah, S., et al. (2014). Magnetic nanoparticle drug delivery systems for targeting tumor. Appl Nanosci, 4, 385–392.
Zhao, Z., Zhou, Z., Bao, J., et al. (2013). Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat Commun, 4, 2266. doi:10.1038/ncomms3266.
Neoh, K. G., & Kang, E. T. (2012). Surface modification of magnetic nanoparticles for stem cell labeling. Soft Matter, 8, 2057–2069.
Wang, Y., Jia, H.-Z., & Han, K. (2013). Theranostic magnetic nanoparticles for efficient capture and in situ chemotherapy of circulating tumor cells. Journal of Materials Chemistry B, 1, 3344–3352.
Gobbo, O. L., Sjaastad, K., & Radomski, M. W. (2015). Magnetic nanoparticles in cancer theranostics. Theranostics, 5(11), 1249–1263.
Thanh, N. T. K. (2012). Magnetic nanoparticles: from fabrication to clinical applications. Boca Raton: CRC Press Taylor and Francis Group.
Huang, P. M., Li, Y., & Sumner, M. E. (2011). Handbook of soil sciences: properties and processes. Boca Raton: CRC Press.
Wu, W., Wu, Z., Yu, T. et al. (2015). Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Science and Technology of Advanced Materials, 16, doi: 10.1088/1468-6996/16/2/023501.
Huan, W., Cheng, C., Yang, Y., et al. (2012). A study on the magnetic and photoluminescence properties of Eun+ and Sm3+ doped Fe3O4 nanoparticles. J Nanosci Nanotechnol, 12, 4621–4634.
Anbarasu, M., Anandan, M., & Chinnasamy, E. (2015). Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim Acta A Mol Biomol Spectrosc, 135, 536–539.
Garzon-Manjon, A., Solano, E., & de la Mata, M. (2015). Induced shape controllability by tailored precursor design in thermal and microwave-assisted synthesis of Fe3O4 nanoparticles. J Nanopart Res, 17, 291. doi:10.1007/s11051-015-3070-x.
Li, C., Wei, R., Xu, Y., et al. (2014). Synthesis of hexagonal and triangular Fe3O4 nanosheets via seed-mediated solvothermal growth. Nano Res, 7(4), 536–543.
Mazario, E., Sanchez-Marcos, J., & Menendez, N. (2014). One-pot electrochemical synthesis of polydopamine coated magnetite nanoparticles. RSC Adv, 4, 48353–48361.
Zhang, W., Zhang, H., & Li, D. (2006). Preparation of Fe3O4 magnetic fluid by one-step method with microemulsion reactor. Frontiers of Chemistry in China, 3, 272–276.
Chin, S. F., Pang, S. C., & Tan, C. H. (2011). Green synthesis of magnetite NPs (via thermal decomposition method) with controllable size and shape. Journal of Materials and Environmental Science, 2(3), 299–302.
Fievet, F., Fievet-Vincent, F., & Lagier, J.-P. (1993). Controlled nucleation and growth of micrometre-size copper particles prepared by the polyol process. J Mater Chem, 3(6), 627–632.
Rao, C. N. R., Müller, A., & Cheetham, A. K. (2006). The chemistry of nanomaterials: synthesis, properties and applications. New York: Wiley.
Grisaru, H., Palchik, O., & Gedanken, A. (2003). Microwave-assisted polyol synthesis of CuInTe2 and CuInSe2 nanoparticles. Inorg Chem, 42(22), 7148–7155.
Abbas, M., Rao, B. P., Naga, S. M., et al. (2013). Synthesis of high magnetization hydrophilic magnetite (Fe3O4) nanoparticles in single reaction—Surfactantless polyol process. Ceram Int, 39(7), 7605–7611.
Coskun, S., Aksoy, B., & Unalan, H. E. (2011). Polyol synthesis of silver nanowires: an extensive parametric study. Crystal Growth Design, 11, 4963–4969.
Kim, C. W., Cha, H. G., Kim, Y. H., et al. (2009). Surface investigation and magnetic behavior of Co nanoparticles prepared via a surfactant-mediated polyol process. J Phys Chem C, 113, 5081–5086.
Komarneni, S., Li, D., & Newalkar, B. (2002). Microwave-polyol process for Pt and Ag nanoparticles. Langmuir, 18, 5959–5962.
Carroll, K. J., Reveles, J. U., & Shultz, M. D. (2011). Preparation of elemental Cu and Ni nanoparticles by the polyol method: an experimental and theoretical approach. J Phys Chem C, 115, 2656–2664.
Mathur, S., Shen, H., ACer, S., et al. (2010). Nanostructured materials and systems: ceramic transactions. New York: Wiley.
Xiaoa, W., Gu, H., Li, D., et al. (2012). Microwave-assisted synthesis of magnetite nanoparticles for MR blood pool contrast agents. J Magn Magn Mater, 324, 488–494.
Mahdavi, M., Ahmad, M. B., & Haron, M. J. (2013). Synthesis, surface modification and characterization of biocompatible magnetic iron oxide nanoparticles for biomedical applications. Molecules, 18, 7533–7548.
Zhao, S.-Y., Lee, D. K., Kim, C. W., et al. (2006). Synthesis of magnetic nanoparticles of Fe3O4 and CoFe2O4 and their surface modification by surfactant adsorption. Bull Kor Chem Soc, 27(2), 237–242.
Rahman, O., Mohapatra, S. C., & Ahmad, S. (2012). Fe3O4 inverse spinal super paramagnetic nanoparticles. Mater Chem Phys, 132, 196–202.
Harris, J. M. (2013). Poly(ethylene glycol) chemistry: biotechnical and biomedical applications. Berlin: Springer.
Jiao, M., Zeng, J., & Jing, L. (2015). Flow synthesis of biocompatible Fe3O4 nanoparticles: insight into the effects of residence time, fluid velocity, and tube reactor dimension on particle size distribution. Chem Mater, 27(4), 1299–1305.
Veronese, F. M. (2009). PEGylated protein drugs: basic science and clinical applications. Switzerland: Birkhäuser Verlag.
FAO/WHO (1980). Evaluation of certain food additives. Twenty-third report of the joint FAO/WHO expert committee on food additives. World Health Organ Tech. Rep. Ser. No. 648.
Fruijtier-Pölloth, C. (2005). Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology, 214(1–2), 1–38.
World Health Organization (2003). Ethylene glycol: human health aspects (Concise International Chemical Assessment Documents (Book 45)).
Acknowledgements
This study was financially supported by grant of Russian Science Foundation (project no. 14-35-00051).
We thank Dr. Peter V. Zolotukhin and Anna A. Belanova (Evolution corporate group) for assistance with cytotoxicity test, and the Joint Research Center “Diagnostics of structure and properties of nanomaterials” of Belgorod National Research University for TGA and XRD measurements and to Dr. Andriy Budnyk (SFedU) for his contribution during preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lastovina, T.A., Efimova, S.A., Kudryavtsev, E.A. et al. Preparation of the Sm3+-Doped Magnetic Nanoparticles via Microwave-Assisted Polyol Synthesis. BioNanoSci. 7, 4–10 (2017). https://doi.org/10.1007/s12668-016-0385-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-016-0385-8