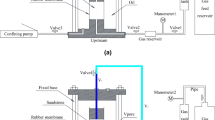
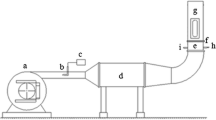
Abstract
The cold permeability of a sinter bed is a complex function of the granules’ effective mean diameter and bed moisture. The binding force provided by moisture before fusion commences crucial in determining the strength of a sinter. However, excess moisture is detrimental to the propagation of the heat front, because it reduces the bed’s permeability. The source of moisture can be either the inherent moisture in the bed or from the incoming gases due to suction in the bottom of the bed. An unsteady-state mathematical model was developed in the current study to describe the moisture transport phenomena during the iron ore sintering. The model was then validated using experimental data from laboratory sintering pot tests. Using the help of the model, the effect of humidity on the moisture characteristics was then investigated. The temperature profiles revealed that the change in the solid temperatures was less than 5% with increasing humid conditions.







Similar content being viewed by others
Abbreviations
- \(\epsilon \) :
-
Void fraction
- \(\lambda \) :
-
Sphericity factor
- \(\mu \) :
-
Viscosity of gas (Pa s)
- \(\rho _{{\mathrm{g}}}\) :
-
Density of gas (\({\hbox {kg/m}}^{3}\))
- \(\rho _{j,{\mathrm{s}}}\) :
-
Density of solid component i (\({\hbox {kg/m}}^{3}\,{\hbox {bed}}\))
- \(\rho _{{\mathrm{s}}}\) :
-
Density of solid (\({\hbox {kg/m}}^{3}\,{\hbox {bed}}\))
- \(\vec {v}\) :
-
Superficial velocity (m/s)
- \(A_{{\mathrm{v}}}\) :
-
Area of particle per unit volume of the bed (\({\hbox {m}}^{2}/{\hbox {m}}^{3}\,{\hbox {bed}}\))
- \(C_{i,{\mathrm{g}}}\) :
-
Concentration of gas component i (\({\hbox {mol/m}}^{3}\,{\hbox {bed}}\))
- \(C_{{\mathrm{p,s/g}}}\) :
-
Specific heat capacity of solid/gas (J/kg K)
- \(D_{{\mathrm{m}}}\) :
-
Mass diffusivity (\({\hbox {m}}^{2}/{\hbox {s}}\))
- \(d_{{\mathrm{p}}}\) :
-
Particle diameter (m)
- \(f_{i/i'}\) :
-
Partition coefficient between solid and gas
- h :
-
Heat transfer coefficient (\({\hbox {W/m}}^{2}\,{\hbox {K}}\))
- \(H_{k}\) :
-
Enthalpy change (J)
- \(k_{w}\) :
-
Mass transfer coefficient for drying/condensation reaction (\({\hbox {m}}^{2}/{\hbox {s}}\))
- \(M_{k}\) :
-
Molar mass (kg/mol)
- P :
-
Bed pressure (Pa)
- \(P_{{\mathrm{H}}_{2}{\mathrm{O}},\,{\mathrm{sat}}}\) :
-
Partial pressure of moisture, saturated (Pa)
- R :
-
Universal gas constant (J/mol K)
- \(R_{k}\) :
-
Reaction rate \({\hbox {mol/m}}^{3}\,{\hbox {s}}\)
- Re:
-
Reynolds number (\({\hbox {Re}}= \rho v d_{{\mathrm{p}}}/ \mu \))
- \(S_{{\mathrm{s/g}}}\) :
-
Source term in solid and gas phase
- Sc:
-
Schmidt number (\( {\hbox {Sc}} =\mu /\rho D_{{\mathrm{m}}}\))
- Sh:
-
Sherwood number
- t :
-
time (s)
- \(T_{{\mathrm{s/g}}}\) :
-
Temperature of solid/gas (K)
- \(w_{{\mathrm{cr}}}\) :
-
Critical moisture content
- Z :
-
Relative humidity
- z :
-
Location along depth of bed (m)
References
Nath N K, Da Silva A J, and Chakraborti N, Steel Res 68 (1997) 285.
Cumming M J, Ironmak Steelmak 17 (1990) 245.
de Castro J A, Sazaki Y, and Yagi J, Mater Res 15 (2012) 848.
Rankin W J, and Roller P W, Trans Iron Steel Inst Japan 25 (1985) 1016.
Wajima M, Hosotani Y, Shibata J, Soma H, and Tashiro K, Tetsu-to-Hagané 68 (1982) 1719.
Venkataramana R, Gupta S S, and Kapur P C, Int J Miner Process 57 (1999) 43.
Ergun S, Chem Eng Prog 48 (1952) 89.
Hinkley J, Waters A G, and Litster J D, Int J Miner Process 42 (1994) 37.
Nozawa K, Morioka K, Kinugasa T, Ano K, and Osuga K, ISIJ Int 53 (2013) 1510.
Zhou H, Zhao J P, Loo C E, Ellis B G, and Cen K F, ISIJ Int 52 (2012) 1550.
Dash I R, and Rose E, IFAC Proc 11 (1978) 151.
Young RW, Proc. Ironmak. Steelmak., 1978 25.
Patisson F, Bellot J P, and Ablitzer D, Metall Trans B 21 (1990) 37.
Pahlevaninezhad M, Emami M D, and Panjepour M, Appl Math Model 40 (2016) 8475.
Arthur J R, Trans Faraday Soc 47 (1951) 164.
Green D W, and Perry R H, Perry’s Chemical Engineers’ Handbook, McGraw-Hill, New York (2008) p. 296
Muller J, de Vries T L, Dippenaar B A, and Vreugdenburg J C, J South African Inst Min Metall 115 (2015) 409.
Zhang B, Zhou J, and Li M, Appl Therm Eng 131 (2018) 70.
Gupta A, Studies on Sintering of Iron Ore, M.Tech Thesis, Indian Institute of Technology, Bombay, India (2012) p. 59.
Sutherland W, Lond Edinb Dublin Philos Mag J Sci 36 (1893) 507.
Wilke C R, J Chem Phys 18 (1950) 517.
Kunii D, Suzuki M, and Ono N, J Chem Eng Japan 1 (1968) 21.
Mitterlehner J, Löffler G, Winter F, Hofbauer H, Schmid H, Zwittag E, Pammer O, and Stiasny H, ISIJ Int 44 (2004) 11.
Shibata J, Math Comput Model 11 (1988) 956.
Patankar S, Numerical Heat Transfer and Fluid Flow: CRC Press, Taylor & Francis Group, LLC. Florida, United States; (1980) p. 77.
Yang W, Ryu C, Choi S, Choi E, Lee D, and Huh W, ISIJ Int 44 (2004) 492.
Venkataraman R, Gupta S S, Kapur P C, and Ramachandran N, Tata Search (India) (1998) 25.
Geiger G H, and Poirier D R, Addison-Wesley Publ. Co., Reading, Mass. (1973) p. 616.
Acknowledgements
The author would like to thank Prof. N. B. Ballal and Prof. N. N. Viswanathan at IIT Bombay for their helpful suggestions and thought-provoking discussions. The people at Ferrous Process Laboratory are also thanked for their help in carrying out the experiments. The author also acknowledges Mr. R. Gupta at McMaster University for his feedback on the manuscript.
Author information
Authors and Affiliations
Contributions
The author is currently a Ph.D. candidate in Materials Science Engineering at McMaster University. The work has been done during graduate studies at Indian Institute of Technology, Bombay.
Corresponding author
Ethics declarations
Conflict of interest
The author reported no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Angshuman Podder: Formerly at Indian Institute of Technology Bombay, Mumbai, India.
Rights and permissions
About this article
Cite this article
Podder, A. Study of Humidity on Moisture Transfer Characteristics in Iron Ore Sintering. Trans Indian Inst Met 74, 1479–1487 (2021). https://doi.org/10.1007/s12666-021-02244-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-021-02244-3