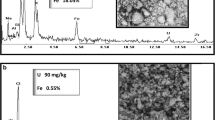
Abstract
The dissolution of uranium dioxide under oxidative alkaline conditions is critically influenced by iron pyrite (FeS2), which is a gangue mineral commonly found in uranium ores. This paper makes an effort in understanding the kinetics of dissolution of UO2 in a co-existing system of UO2 and FeS2 under the lixiviant combination of Na2CO3 and NaHCO3–O2 at elevated temperature and pressure. Dissolution experiments were carried out in a laboratory batch autoclave reactor using synthetic mixtures of minerals consisting of UO2, FeS2 (reactive gangue—varied from 1 to 6 %), silica (inert gangue) and calcite (inert gangue). The kinetic profiles indicated that the rate of dissolution of UO2 increased with initial increase in FeS2 content in the feed and decreased when the FeS2 weight increased beyond 3 %. The dissolution phenomenon was analysed using scanning electron microscopy and X-ray diffraction studies.









Similar content being viewed by others
References
McKay A D, and Miezitis Y, Australia’s uranium resources, geology and development of deposits. In: AGSO-Geoscience Australia, Mineral Resource Report 1, (2001) p 184.
Merrit R C. The Extractive Metallurgy of Uranium, Colorado School of Mines Research Institute, Golden Colorado, United States Atomic Energy Commission, Colorado (1971) p 35.
Anon, Manual on Laboratory Testing of Uranium Ore Processing, Technical reports series no. 313, International Atomic Energy Agency (1990) p 45.
Anon, Uranium Extraction Technology, Technical reports series no. 359, International Atomic Energy Agency, Vienna (1993) p 80.
Du Preez, J G H, Morris D C, and Van Vuuren C P J, Hydrometallurgy 6 (1981) 197.
Mattus Jr A J, and Torma A E, Hydrometallurgy 5 (1980) 179.
Du Preez J G H, Morris D C, and Van Vuuren C P J, Hydrometallurgy 6 (1981) 203.
Ciminelli V S T, and Osseo-Asare K, Metall Mater Trans B, 26B (1995) 209.
Guilinger T R, Schechter R S, and Lake L W, Ind Eng Chem Res 26 (1987) 824.
Joshi J B, Shah Y T, and Albal R S, Ind Eng Chem Process Des Dev 21 (1982) 594.
Hossain M M, Ekeroth E, and Jonsson M, J Nucl Mater 358 (2006) 202.
Choy C C, Korfiatis G P, and Meng X, J Hazard Mater 136 (2006) 53.
Parihar P S, in Proc Conference on Sustainable Mining and the United Nations Framework Classification (UNFC) UNFC workshop, Ministry of Mines, Government of India. New Delhi (2013).
Chaki A, Panneerselvam A, and Chavan S J, in Proc International Symposium on Uranium Production and Raw Materials for the Nuclear Fuel Cycle Supply and Demand, Economics, the Environment and Energy Security, International Atomic Energy Agency (IAEA-CN-128), Vienna (2005), p 183.
Rao K A, Sreenivas T, Vinjamur, and Suri A K, Hydrometallurgy 146 (2014) 119.
Anand Rao K, and Suri A K, Hydrometallurgy 141 (2014) 67.
Suri A K, Padmanabhan N P H, Sreenivas T, Anand Rao K, Singh A K, Shenoy K T, Mishra T, and Ghosh S K, in Proc IAEA Technical Meeting on Low-grade uranium deposits, International Atomic Energy Agency, Vienna (2010).
Padmanabhan N P H, Sreenivas T, Anand Rao K, Manmadha Rao M, Rajan K C, Serajuddin Md, and Karthikayini P, Process Development Studies for the Recovery of Uranium from the Gogi Limestone-type Ore, An Unpublished Internal Report, Mineral Processing Division, Bhabha Atomic Research Centre, Hyderabad (2010) 104.
Fogler H S. Elements of Chemical Reaction Engineering, 3rd ed. (ed) Prentice-Hall, Richmond (2004), p 811.
Levenspiel O, Chemical Reaction Engineering, (ed) Wiley, New York (2001).
Lundstrom M, Aromaa J, and Forsen O, Physicochem Probl Miner Process 46 (2011) 263.
Gavrilescu M, Pavel LV, and Cretescu I, J Hazard Mater, 163 (2009) 475.
Hiskey J B, Institution of Mining and Metallurgy (1979) C 145.
DePablo J, Casa I, Gimenez J, Molera M, Rovira M, Duro L, and Bruno J, Geochim Cosmochim Acta 63 (1999) 3097.
Grandstaff D E, Econ Geol 71 (1976).
Reddy L S R, Unpublished Internal Report of Atomic Minerals Directorate for Exploration and Research, Department of Atomic Energy, Government of India, (Report No.: AMD/PET/HYD/ODG - 17/2007) (2007).
Roy M, Visakha Sci J 4 (2000) 67.
Roy M, and Dhana Raju R, J Appl Geochem 1 (1999) 53.
Suri A K, Sreenivas T, Anand Rao K, Rajan K C, Srinivas K, Singh A K, Shenoy K T, Mishra T, Padmanabhan N P H, and Ghosh S K, Miner Process Extr Metall (Trans Inst Min Metall Sec.C) 123 (2014) 104.
Latha A, Sharma A K, Vishwamohan K, Unpublished Internal Report of Atomic Minerals Directorate for Exploration and Research, Department of Atomic Energy, Government of India, (Report No.: AMD/PET/12/2009) (2009).
Latha A, Sharma A K, Fahmi S, and Shivakumar K, J Appl Geochem 14 (2012) 316.
Srikantappa C, and Govindaiah S, Indian Miner 44 (2010) 1.
Acknowledgments
The authors convey their sincere thanks to their colleagues in chemical laboratory of Mineral Processing Division and Shri Bhaskar Paul of Material Processing Division of Bhabha Atomic Research Centre for the wet chemical analyses and scanning electron microscopy respectively. They also acknowledge the services of the XRD Laboratory of Atomic Minerals Directorate for Exploration and Research in carrying out XRD analyses. The authors also thank Dr. J.K. Chakravartty, Director, Materials Group, Bhabha Atomic Research Centre for his encouragement during the investigations. The authors are thankful for the valuable suggestions from two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anand Rao, K., Sreenivas, T., Vinjamur, M. et al. Kinetics of Alkaline Leaching of UO2 and FeS2 in Co-existing System. Trans Indian Inst Met 69, 23–31 (2016). https://doi.org/10.1007/s12666-015-0615-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-015-0615-8