Abstract
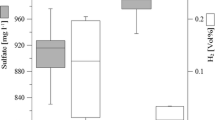
Two geological formations at the CO2 storage pilot site in Ketzin (Germany) were geochemically and microbiologically characterized to further evaluate changes resulting from CO2 injection. Well fluids were collected from both Stuttgart (storage formation, ~650 m depth) and Exter Formations (~400 m depth, overlying the caprock) either through pump tests or downhole samplings. Rock samples were retrieved during a deep drilling into the Exter Formation and primarily comprised quartz, ferrous dolomite or ankerite, calcite, analcime, plagioclase and clay minerals, as determined through X-ray diffraction analyses. In the rocks, the total organic carbon (TOC), which potentially contributes to microbial growth, was mostly below 1000 mg kg−1. The geochemical characterization of fluids revealed significant differences in the ionic composition between both formations. The microbial characterization was performed through fluorescence in situ hybridization and 16S rRNA gene fingerprinting. In the fluids obtained from the Stuttgart Formation, the microbial activity was affected by the relatively high TOC, introduced by the organic drill mud. The total cell counts were approximately 106 cells mL−1. The microbial community was characteristic of a saline deep biosphere environment enriched through increased carbon availability, with sulfate-reducing bacteria as the most abundant microorganisms (up to 60 % of total cells). Species belonging to halophilic/halotolerant Proteobacteria and Firmicutes were primarily detected. In Exter Formation rocks, Proteobacteria and Actinobacteria were detected. These data provide an explicit reference to further evaluate environmental changes and community shifts in the reservoir during CO2 storage and provide information for evaluating the storage efficiency and reliability.







Similar content being viewed by others
References
Aldén L, Demoling F, Bååth E (2001) Rapid method of determining factors limiting bacterial growth in soil. Appl Environ Microb 67:1830–1838. doi:10.1128/AEM.67.4.1830-1838.2001
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analysing mixed microbial populations. Appl Environ Microb 56(6):1919–1925
Baker BJ, Moser DP, MacGregor BJ, Fishbain S, Wagner M, Fry NK, Jackson B et al (2003) Related assemblages of sulphate-reducing bacteria associated with ultradeep gold mines of south Africa and deep basalt aquifers of Washington state. Environ Microbiol 5:267–277. doi:10.1046/j.1462-2920.2003.00408.x
Bassam BJ, Caetano-Anolles G, Gresshoff PM (1991) Fast and sensitive staining of DNA in polyacrylamide gels. Anal Biochem 196:80–83
Basso O, Lascourreges JF, Jarry M, Margot M (2005) The effect of cleaning and disinfecting the sampling well on the microbial communities of deep subsurface water samples. Environ Micobiol 7:13–21. doi:10.1111/j.1462-2920.2004.00660.x
Basso O, Lascourreges JF, Le Borgne F, Le Goff C, Margot M (2009) Characterization by culture and molecular analysis of the microbial diversity of a deep subsurface gas storage aquifer. Res Microbiol 160(2):107–116. doi:10.1016/j.resmic.2008.10.010
Bauer S, Beyer C, Dethlefsen F, Dietrich P, Duttmann R, Ebert M, Feeser V, Görke U, Köber R, Würdemann H, Dahmke A (2013) Impacts of the use of the geological subsurface for energy storage: an investigation concept. Environ Earth Sci 70:3935–3943. doi:10.1007/s12665-013-2883-0
Beech IB, Sunner JA, Hiraoka K (2005) Microbe-surface interactions in biofouling and biocorrosion processes. Int Microbiol 8(3):157–168
Berner A (1970) Sedimentary pyrite formation. Am J Sci 268:1–23. doi:10.2475/ajs.268.1.1
Bock S, Förster H, Meier A, Pudlo D, Förster A, Gaupp R (2013) Impact of 4-year CO2 injection on reservoir-rock integrity at the CO2 pilot site Ketzin (Germany). Abstracts #H21L-02 presented at 2013 Fall Meeting, AGU, San Francisco, Calif., 9–13 Dec
Boivin-Jahns V, Ruimy R, Bianchi A, Daumas S, Christen R (1996) Bacterial diversity in a deep-subsurface clay environment. Appl Environ Microb 62(9):3405–3412
Brockman FJ, Kieft TL, Fredrickson JK, Bjornstad BN, Li SW, Spangenburg W, Long PE (1992) Microbiology of vadose zone paleosols in South-Central Washington State. Microbiol Ecol 23:279–301
Crombie DJ, Moody GJ, Thomas JDR (1980) Corrosion of iron by sulfate-reducing bacteria. Chem Ind London 21(6):500–505
Czarnetzki AB, Tebbe CC (2004) Diversity of bacteria associated with collembola—a cultivation-independent survey based on PCR-amplified 16S rRNA genes. FEMS Microbiol Ecol 49:217–227. doi:10.1016/j.femsec.2004.03.007
Daims H, Brühl A, Amann R, Schleifer K-H, Wagner M (1999) The domain-specific probe EUB338 is insufficient fort the detection of all bacteria; development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22(3):434–444. doi:10.1016/S0723-2020(99)80053-8
Dohrmann AB, Tebbe CC (2004) Microbial community analysis by PCR-single-strand conformation polymorphism (PCR-SSCP). In: Kowalchuk GA, Bruijn FJ de, Head IM, Akkermans AD, van Elsas JD (eds) Molecular microbial ecology manual, 2nd edn. Kluwer Academic Publisher, Dodrecht 3(16):809–838
Enning D, Garrelfs J (2014) Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl Environ Microb 80(4):1226–1236. doi:10.1128/AEM.02848-13
Fischer S, Liebscher A, Wandrey M, the CO2SINK Group (2010) CO2-brine-rock interaction—first results of long-term exposure experiments at in situ P–T conditions of the Ketzin CO2 reservoir. Chem Erde-Geochem 70(S3):155–164. doi:10.1016/j.chemer.2010.06.001
Ford T, Mitchell R (1990) The ecology of microbial corrosion. Adv Microb Ecol 11:231–262
Förster A, Giese R, Juhlin C, Norden B, Springer N, the CO2SINK Group (2009) The geology of the CO2SINK site: from regional scale to laboratory scale. Energy Proc 1(1):2911–2918. doi:10.1016/j.egypro.2009.02.066
Förster A, Schöner R, Förster HJ, Norden B, Blaschke AW, Luckert J, Beutler G, Gaupp R, Rhede D (2010) Reservoir characterization of a CO2 storage aquifer: the upper triassic stuttgart formation in the Northeast German Basin. Mar Petrol Geol 27:2156–2172. doi:10.1016/j.marpetgeo.2010.07.010
Fredrickson JK, Balkwill DL (2006) Geomicrobial processes and biodiversity in the deep terrestrial subsurface. Geomicrobiol J 23(6):345–356. doi:10.1080/01490450600875571
Fry NK, Friedrickson JK, Fishbain S, Wagner M, Stahl D (1997) Population structure of microbial communities associated with two deep, anaerobic, alkaline aquifers. Appl Environ Microb 63(4):1498–1504
Glasauer S, Weidler PG, Langley S, Beveridge TJ (2003) Controls on Fe reduction and mineral formation by a subsurface bacterium. Geochim Cosmochim Acta 67:1277–1288. doi:10.1016/S0016-7037(02)01199-7
Goldscheider N, Hunkeler D, Rossi P (2006) Review: microbial biocenoses in pristine aquifers and an assessment of investigative methods. Hydrogeol J 14(6):926–941. doi:10.1007/s10040-005-0009-9
Gulliver DM, Lowry GV, Gregory KB (2014a) CO2 concentration and pH alters subsurface microbial ecology at reservoir temperature and pressure. RSC Adv 4:17443–17453. doi:10.1039/C4RA02139H
Gulliver DM, Lowry GV, Gregory KB (2014b) Effect of CO2(aq) exposure on a freshwater aquifer microbial community from simulated geologic carbon storage leakage. Environ Sci Technol Lett 1(12):479–483. doi:10.1021/ez500337v
Hao OJ, Chen JM, Huang L, Buglass RL (1996) Sulfate-reducing bacteria. Crit Rev Env Sci Tec 26(2):155–187. doi:10.1080/10643389609388489
Hazen TC, Jimenez L, Lopez de Victoria G, Fliermans CB (1991) Comparison of bacteria from deep subsurface sediment and adjacent groundwater. Microbial Ecol 22(1):293–304. doi:10.1007/BF02540231
Iverson VP (1987) Microbial corrosion of metals. Adv Apl Microbiol 32(1):1–36. doi:10.1016/S0065-2164(08)70077-7
Jakobsen TF, Kjeldsen KU, Ingvorsen K (2006) Desulfohalobium utahense sp. nov., a moderately halophilic, sulfate-reducing bacterium isolated from Great Salt Lake. Int J Syst Evol Micr 56(9):2063–2069. doi:10.1099/ijs.0.64323-0
Jørgensen BB, Boetius A (2007) Fast and famine—microbial life in the deep-sea bed. Nature 5:770–781. doi:10.1038/nrmicro1745
Kallmeyer J, Mangelsdorf K, Cragg B, Horsfield B (2006) Techniques for contamination assessment during drilling for terrestrial subsurface sediments. Geomicrobiol J 23:227–239. doi:10.1080/01490450600724258
Kasina M, Bock S, Würdemann H, Pudlo D, Picard A, Lichtschlag A, März Ch, Wagenknecht L, Wehrmann LM, Vogt C, Ferdelman TG, Meister P. Mineralogical and geochemical analysis of Fe-phases in drill-cores from the Triassic Stuttgart Formation at Ketzin CO2 storage site before CO2 arrival. Environ Earth Sci (submitted)
Kotelnikova S (2002) Microbial production and oxidation of methane in deep subsurface. Earth-Sci Rev 58:367–395. doi:10.1016/S0012-8252(01)00082-4
Kouduka M, Suko T, Morono Y, Inagaki F, Ito K, Suzuki Y (2012) A new DNA extraction method by controlled alkaline treatments from consolidated subsurface sediments. FEMS Microbiol Lett 326:47–54. doi:10.1111/j.1574-6968.2011.02437.x
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Lavania M, Sarma PM, Mandal AK, Cheema S, Lai B (2011) Efficacy of natural biocide on control of microbial induced corrosion in oil pipelines mediated by Desulfovibrio vulgaris and Desulfovibrio gigas. J Environ Sci 23(8):1394–1402. doi:10.1016/S1001-0742(10)60549-9
Lee W, Lewandowski Z, Nielsen PH, Hamilton WA (1995) Role of sulphate-reducing bacteria in corrosion of mild steel: a review. Biofouling 8(3):165–194. doi:10.1080/08927019509378271
Lerm S, Westphal A, Miethling-Graff R, Alawi M, Seibt A, Wolfgramm M, Wurdemann H (2013) Thermal effects on microbial composition and microbiologically induced corrosion and mineral precipitation affecting operation of a geothermal plant in a deep saline aquifer. Extremophiles 17(2):311–327. doi:10.1007/s00792-013-0518-8
Manz W, Amann R, Ludwig W, Wagner M, Schleifer K-H (1992) Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst Appl Microbiol 15(4):593–600. doi:10.1016/S0723-2020(11)80121-9
Manz W, Eisenbrecher M, Neu TR, Szewzyk U (1998) Abundance and spatial organization of Gram-negative sulfate-reducing bacteria in activated sludge investigated by in situ probing with specific 16S rRNA targeted oligonucleotides. FEMS Microbiol Ecol 25(1):43–61. doi:10.1111/j.1574-6941.1998.tb00459.x
Martens S, Kempka T, Liebscher A, Lüth S, Möller F, Myrttinen A, Norden B, Schmidt-Hattenberger C, Zimmer M, Kühn M, the Ketzin Group (2012) Europe’s longest-operating on-shore CO2 storage site at Ketzin, Germany: a progress report after three years of injection. Environ Earth Sci 67(2):323–334. doi:10.1007/s12665-012-1672-5
Martens S, Liebscher A, Möller F, Henninges J, Kempka T, Lüth S, Norden B, Prevedel B, Szisybalski A, Zimmer M, Kühn M, the Ketzin Group (2013) CO2 Storage at the Ketzin Pilot Site, Germany: fourth year of injection, monitoring, modelling and verification. En Proc 37:6434–6443. doi:10.1016/j.egypro.2013.06.573
Meier H, Amann R, Ludwig W, Schleifer K-H (1999) Specific oligonucleotide probes for in situ detection of a major group of gram-positive bacteria with low DNA G+C content. Syst Appl Microbiol 22(2):186–196. doi:10.1016/S0723-2020(99)80065-4
Metz B, Davidson O, de Coninck HC, Loos M, Meyer LA (eds) (2005) IPCC Special report on carbon dioxide capture and storage. Cambridge Univ Press, Cambridge
Morozova D, Wandrey M, Zimmer M, Pilz P, Zettlitzer M, Würdemann H, the CO2SINK Group (2010) Monitoring of the microbial community composition in saline aquifers during CO2 sequestration by fluorescence in situ hybridisation. Int J Greenh Gas Control 4:981–989. doi:10.1016/j.ijggc.2009.11.014
Morozova D, Alawi M, Shaheed M, Krüger M, Kock D, Würdemann H (2011) The influence of microbial activity on rock fluid interaction: baseline characterization of deep microbial biosphere for enhanced gas recovery in the Altmark natural gas reservoir. En Proc 4:4633–4640. doi:10.1016/j.egypro.2011.02.423
Morozova D, Let D, Würdemann H (2013) Analysis of the microbial community from a saline aquifer prior to CO2 injection in Ketzin using improved Fluorescence in situ Hybridisation method. Energy Proc 40:276–284. doi:10.1016/j.egypro.2013.08.032
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Neef A (1997) Anwendung der in situ Einzell-Identifizierung von Bakterien zur Populationsanalyse in komplexen mikrobiellen Biozinösen. Ph.D. thesis, Technische Universität München
Norden B, Klapperer S (2011) Geologischer Abschlußbericht der Bohrung Hy Ktzi P 300/2011. Deutsches Geoforschungzentrum GFZ, Helmholtz Zentrum Potsdam
Norden B, Förster A, Vu-Hoang D, Marcelis F, Springer N, Le Nir I (2010) Lithological and petrophysical core-log interpretation in CO2SINK, the european CO2 onshore research store and verification project. SPE Reserv Eval Eng 13(2):179–192. doi:10.2118/115247-PA
Oren A (2008) Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst 4:2. doi:10.1186/1746-1448-4-2
Pankhania IP (1988) Hydrogen metabolism in sulfate-reducing bacteria and its role in anaerobic corrosion. Biofouling 1(1):27–47. doi:10.1080/08927018809378094
Pedersen K, Hallbeck L, Arlinger J, Erlandson AC, Jahromi N (1997) Investigation of the potential for microbial contamination of deep granitic aquifers during drilling using 16S rRNA gene sequencing and culturing method. J Microbiol Meth 30:179–192. doi:10.1016/S0167-7012(97)00066-3
Pellizzari L, Neumann D, Alawi M, Voigt D, Norden B, Würdemann H (2013) Use of tracers to assess drill mud penetration depth into sandstone rock cores during deep drilling: method development and application. Environ Earth Sci 70:3727–3738. doi:10.1007/s12665-013-2715-2
Pernthaler J, Glöckner FO, Schönhuber W, Amann R (2001) Fluorescence in situ hybridization. In: Paul J (ed) Methods in microbiology: marine microbiology, vol 30. Academic Press Ltd, London
Pellizzari L, Kasina M, Lienen T, Würdemann H. Influence of drill mud on the microbial communities of sandstone rocks and well fluids at the Ketzin pilot site for CO2 storage. Enviro Earth Sci (submitted)
Prevedel B, Wohlgemuth L, Legarth B, Henninges J, Schütt H, Schmidt-Hattenberger C, Norden B, Förster A, Hurter S (2009) The CO2SINK boreholes for geological CO2 -storage testing. Energy Proc 1:2087–2094. doi:10.1016/j.egypro.2009.01.272
Ramsing NB, Fossing H, Ferdelman TG, Andersen F, Thamdrup B (1996) Distribution of bacterial populations in a stratified fjord (Mariager Fjord, Denmark) quantified by in situ hybridization and related to chemical gradients in the water column. Appl Environ Microb 62(4):1391–1404
Sahl JW, Schmidt R, Swanner ED, Mandernack KW, Templeton AS, Kieft TL, Smith RL, Sanford WE, Callighan RL, Mitton JB, Spear JR (2008) Subsurface microbial diversity in deep-granitic-fracture water in Colorado. Appl Environ Microb 74:143–152. doi:10.1128/AEM.01133-07
Salter SJ, Cox MJ, Turek EJ, Calus SJ, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman NJ, Walker AW (2014) Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 12:87. doi:10.1186/s12915-014-0087-z
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Scherf AK, Zetzl C, Smirnova I, Zettlitzer M, Vieth-Hillebrand A, the CO2SINK-group (2011) Mobilisation of organic compounds from reservoir rocks through the injection of CO2—comparison of baseline characterization and laboratory experiments. En Proc 4:4524–4531. doi:10.1016/j.egypro.2011.02.409
Schilling F, Borm G, Giese R, Schmidt-Hattenberger C, Wohlgemuth L, Würdemann H, Zimmer M, the CO2SINK Group (2008) CO2 injection at Ketzin. In: Kukla P, Littke R (eds) Schriftenreihe der Deutschen Gesellschaft für Geowissenschaften, Heft 60, International Conference and 106th Annual Meeting of the Deutsche Gesellschaft für Geowissenschaften e.V. (DGG) and 98th Annual Meeting of the Geologische Vereinigung e.V. (GV) p. 126
Schilling F, Borm G, Würdemann H, Möller F, Kühn M (2009) Status report on the First European on-shore CO2 storage site at Ketzin (Germany). Energy Proc 1:2029–2035. doi:10.1016/j.egypro.2009.01.264
Schoonen MAA (2004) Mechanisms of sedimentary pyrite formation. GSA Special Papers 379:117–134. doi:10.1130/0-8137-2379-5.117
Schultze-Lam S, Fortin D, Davis B, Beveridge TJ (1996) Mineralization of bacterial surfaces. Chem Geol 132:171–181. doi:10.1016/S0009-2541(96)00053-8
Shimizu S, Akiyama M, Naganuma T, Fujioka M, Nako M, Ishijima Y (2007) Molecular characterization of microbial communities in deep coal seam groundwater of northern Japan. Geobiology 5(4):423–433. doi:10.1111/j.1472-4669.2007.00123.x
Sorokin DY, Tourova TP, Mussmann M, Muyzer G (2008) Dethiobacter alkaliphilus gen. nov sp nov., and Desulfurivibrio alkaliphilus gen. nov sp nov.: two novel representatives of reductive sulfur cycle from soda lakes. Extremophiles 12:431–439. doi:10.1007/s00792-008-0148-8
Stahl DA, Amann D (1991) Development and application of nucleic acid probes. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester, pp 205–248
Stubner S, Meuser K (2000) Detection of Desulfotomaculum in an Italian rice paddy soil by 16S ribosomal nucleic acid analyses. FEMS Microbiol Ecol 34(1):73–80. doi:10.1111/j.1574-6941.2000.tb00756.x
Takai K, Moser DP, DeFlaun M, Onstott TC, Fredrickson JK (2001) Archaeal diversity in waters from deep South African gold mines. Appl Environ Microb 67:5750–5760. doi:10.1128/AEM.67.21.5750-5760.2001
Wallner G, Amann R, Beisker W (1993) Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14(2):136–143. doi:10.1002/cyto.990140205
Wandrey M, Morozova D, Zettlitzer M, Würdemann H, the CO2SINK Group (2010) Assessing drilling mud and technical fluid contamination in rock core and brine samples intended for microbiological monitoring at the CO2 storage site in Ketzin using fluorescent dye tracers. Int J Greenh Gas Control 4(6):972–980. doi:10.1016/j.ijggc.2010.05.012
Wandrey M, Pellizzari L, Zettlitzer M, Würdemann H (2011) Microbial community and inorganic fluid analysis during CO2 storage within the frame of CO2SINK—long-term experiments under in situ conditions. Energy Proc 4:3651–3657. doi:10.1016/j.egypro.2011.02.296
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Welch SA, Barker WW, Banfield JF (1999) Microbial extracellular polysaccharides and plagioclase dissolution. Geochim Cosmochim Ac 63(9):1405–1419. doi:10.1016/S0016-7037(99)00031-9
Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95:6578–6583. doi:10.1073/pnas.95.12.6578
Whittleston RA, Stewart DI, Mortimer RJG, Burke IT (2013) Enhancing microbial iron reduction in hyperalkaline, chromium contaminated sediments by pH amendment. Appl Geochem 28:135–144. doi:10.1016/j.apgeochem.2012.10.003
Wiese B, Böhner J, Enachescu C, Würdemann H, Zimmermann G (2010) Hydraulic characterisation of the Stuttgart formation at the pilot test site for CO2 storage, Ketzin, Germany. Int J Greenh Gas Control 4(6):960–971. doi:10.1016/j.ijggc.2010.06.013
Wiese B, Zimmer M, Nowak M, Pellizzari L, Pilz P (2013) Well-based hydraulic and geochemical monitoring of the above zone of the CO2 reservoir at Ketzin, Germany. Environ Earth Sci 70:3709–3726. doi:10.1007/s12665-013-2744-x
Würdemann H, Möller F, Kühn M, Heidung W, Christensen NP, Borm G, Schilling FR (2010) CO2SINK-From site characterisation and risk assessment to monitoring and verification: one year of operational experience with the field laboratory for CO2 storage at Ketzin, Germany. Int J Greenh Gas Control 4(6):938–951. doi:10.1016/j.ijggc.2010.08.010
Zettlitzer M, Möller F, Morozova D, Lokay P, Würdemann H, the CO2SINK Group (2010) Re-establishment of the proper injectivity of the CO2-injection well Ktzi 201 in Ketzin, Germany. Int J Greenh Gas Control 4:952–959. doi:10.1016/j.ijggc.2010.05.006
Acknowledgments
We would like to thank all partners in the Ketzin projects and CO2 storage center for their continued support and contributions. Special thanks go to EEW (Erdoel-Erdgas Workover GmbH) and Dr. Andrea Seibt (BWG) for their most valuable advice and operational support during the downhole sampling and to Dr. Maren Wandrey for her support during the pump test at the Ktzi 202 well and for the corresponding fluorescein measurements. Dr. Hannah Halm and Dr. Tobias Lienen (GFZ German Research Centre for Geosciences, Potsdam) are acknowledged for critical reading of the manuscript. This research was funded by the European Commission, the Federal Ministry of Economics and Technology and the Federal Ministry of Education and Research in the frame of the CO2SINK project and by the Federal Ministry for Education and Research within the GEOTECHNOLOGIEN Program in the framework of CO2MAN (CO2 Reservoir Management 03G0760A-F).
Author information
Authors and Affiliations
Corresponding author
Additional information
Linda Pellizzari and Daria Morozova have contributed to the manuscript equally.
Rights and permissions
About this article
Cite this article
Pellizzari, L., Morozova, D., Neumann, D. et al. Comparison of the microbial community composition of pristine rock cores and technical influenced well fluids from the Ketzin pilot site for CO2 storage. Environ Earth Sci 75, 1323 (2016). https://doi.org/10.1007/s12665-016-6111-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-6111-6