Abstract
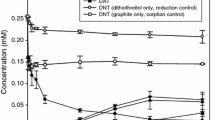
The objective of this study is to investigate the fate of redox-sensitive nitro compounds in the presence of black carbon (BC) materials in electron-rich subsurface environments. The ability of various types of BC was examined, including graphite, carbon nanotubes, chemically converted graphene, activated carbon, diesel soot, and biochar, to promote the reduction of 2,4-dinitrotoluene, pendimethalin, and trifluralin by hydrogen sulfide, a naturally occurring reductant, through batch experiments. Compared to removal in sorption control experiments with BC materials and direct transformation with hydrogen sulfide, the presence of BC markedly enhanced the reduction of nitro compounds by hydrogen sulfide, indicating that BC can be an electron-transfer mediator in the presence of hydrogen sulfide. Possible mechanisms and environmental implications of BC-mediated reduction reactions in soils and sediments are discussed.








Similar content being viewed by others
References
Accardi-Dey A, Gschwend PM (2002) Assessing the combined roles of natural organic matter and black carbon as sorbents in sediments. Environ Sci Technol 36:21–29
Amezquita-Garcia HJ, Razo-Flores E, Cervantes FJ, Rangel-Mendez JR (2013) Activated carbon fibers as redox mediators for the increased reduction of nitroaromatics. Carbon 55:276–284
Barrows SE, Cramer CJ, Truhlar DG, Evolitz MS, Weber EJ (1996) Factors controlling regioselectivity in the reduction of polynitroaromatics in aqueous solution. Environ Sci Technol 30:3028–3038
Chiu CT, Kile DE (1998) Deviations from sorption linearity on soils of polar and nonpoloar organic compounds at low relative concentrations. Environ Sci Technol 32:338–343
Cornelissen G, Gustafsson Ö (2005) Importance of unburned coal carbon, black carbon, and amorphous organic carbon to phenanthrene sorption in sediments. Environ Sci Technol 39:764–769
Cornelissen G, Gustafsson Ö, Bucheli TD, Jonker MTO, Koelmans AA, van Noort PCM (2005) Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol 39:6881–6895
Dusek U, Reischl GP, Hitzenberger R (2006) CCN activation of pure and coated carbon black particles. Environ Sci Technol 40:1223–1230
Goldberg ED (1985) Black carbon in the environment. Wiley, New York
Gustafsson Ö, Gschwend PM (1998) The flux of black carbon to surface sediments on the New England continental shelf. Geochim Cosmochim Acta 62:465–472
Gustafsson Ö, Haghseta F, Chan C, MacFarlane J, Gschwend PM (1997) Quantification of the dilute sedimentary soot phase: implications for PAH speciation and bioavailability. Environ Sci Technol 31:203–209
Keiluweit M, Kleber M (2009) Molecular-level interaction in soils and sediments: the role of aromatic π-systems. Environ Sci Technol 43:3421–3429
Kemper JM, Ammar E, Mitch WA (2008) Abiotic degradation of hexahydro-1,3,5-trinitro-1,3,5-triazine in the presence of hydrogen sulfide and black carbon. Environ Sci Technol 42:2118–2123
Lohmann R, MacFarlane JK, Gschwend PM (2005) Importance of black carbon to sorption of native PAHs, PCBs, and PCDDs in Boston and New York harbor sediments. Environ Sci Technol 39:141–148
Middelburg JJ, Nieuwenhuize J, Van Breugel P (1999) Black carbon in marine sediments. Mar Chem 65:245–252
Nguyen TH, Sabbah I, Ball WP (2004) Sorption nonlinearity for organic contaminants with diesel soot: method development and isotherm interpretation. Environ Sci Technol 38:3595–3603
Oh SY, Cha DK, Chiu PC (2002) Graphite-mediated reduction of 2,4-dinitrotoluene with elemental iron. Environ Sci Technol 36:2178–2184
Oh SY, Cha DK, Kim BJ, Chiu PC (2004) Reduction of nitroglycerin with elemental iron: pathway, kinetics, and mechanisms. Environ Sci Technol 38:3723–3730
Oh SY, Cha DK, Kim BJ, Chiu PC (2005) Transformation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX), octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) and methylenedinitramine (MDNA) with elemental iron. Environ Toxicol Chem 24:2812–2819
Oh SY, Chiu PC (2009) Graphite- and soot-mediated reduction of 2,4-dinitrotoluene and hexahydro-1,3,5-trinitro-1,3,5-triazine. Environ Sci Technol 43:6983–6988
Oh SY, Son JG, Lim OT, Chiu PC (2012) The role of black carbon as a catalyst for environmental redox transformation. Environ Geochem Health 34:105–113
Oh SY, Son JG, Hur SH, Chung JS, Chiu PC (2013a) Black carbon-mediated reduction of 2,4-dinitrotoluene by dithiothreitol. J Environ Qual 42:815–821
Oh SY, Son JG, Chiu PC (2013b) Biochar-mediated reductive transformation of nitro herbicides and explosives. Environ Toxicol Chem 32:501–508
Rickard D, Luther GW (2007) Chemistry of iron sulfides. Chem Rev 107:514–562
Sander M, Pignatello JJ (2005) Characterization of charcoal adsorption sites for aromatic compounds: insights drawn from single-solute and bi-solute competitive experiments. Environ Sci Technol 39:1606–1615
Schmidt MWI, Noack AG (2000) Black carbon in soils and sediments: analysis, distribution, implications, and current challenges. Glob Biogeochem Cycles 14:777–793
Schwarzenbach RP, Gschwend PM, Imboden DM (2002) Environmental organic chemistry, 2nd edn. Wiley, New York
Thorseon WA, Cope WG, Shea D (2004) Bioavailability of PAHs: effects of soot carbon and PAH source. Environ Sci Technol 38:2029–2037
Xu W, Dana KE, Mitch WA (2010) Black carbon-mediated destruction of nitroglycerin and RDX by hydrogen sulfide. Environ Sci Technol 44:6409–6415
Xu W, Pignatello JJ, Mitch WA (2013) The role of black carbon conductivity in mediating hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) transformation on carbon surfaces by sulfides. Environ Sci Technol 47:7129–7136
Ye J, Chiu PC (2006) Transport of atomic hydrogen through graphite and its reaction with azoaromatic compounds. Environ Sci Technol 40:3959–3964
Van der Zee FP, Bisschops IAE, Lettinga G, Field JA (2003) Activated carbon as an electron acceptor and redox mediator during the anaerobic biotransformation of azo dyes. Environ Sci Technol 37:402–408
Yu X, Gong W, Liu X, Shi L, Han X, Bao H (2011) The use of carbon black to catalyze the reduction of nitrobenzenes by sulfides. J Hazard Mater 198:340–346
Zhu D, Pignatello JJ (2005) Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model. Environ Sci Technol 39:2033–2041
Acknowledgments
This research was supported by the 2014 research fund of University of Ulsan, South Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, SY., Son, JG. & Chiu, P.C. Black carbon-mediated reductive transformation of nitro compounds by hydrogen sulfide. Environ Earth Sci 73, 1813–1822 (2015). https://doi.org/10.1007/s12665-014-3535-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3535-8