Abstract
Purpose
Ever increasing food waste production has promoted anaerobic digestion and composting for its proper management, producing a relevant amount of recycled organic waste (OW) for possible agricultural uses. However, little is known regarding soil carbon management using this type of OW.
Methods
In this study, an anaerobic digestate from the wet digestion of food waste (WETD), and one from the dry-batch process (DRYD), along with their respective composts (WETC and DRYC), were utilized in a pot test over two growing cycles (84 + 84 days), with and without mineral nitrogen (N) fertilization, and were compared with a bio-waste compost (BWC) and a chemical reference (Chem). At the end of the two growth cycles (days 84 and 168), the ryegrass dry biomass (DW) and the N uptake were assessed.
Results
The pot soil was analyzed for soil organic carbon (SOC) and the potassium permanganate (KMnO4) oxidizable fraction (CL) as well as δ13C and Δ13C. At day 84, the SOC (g kg− 1) was the highest in DRYD and DRYC (8.53) > WETD and WETC (7.71) = BWC (7.86) > Chem (6.68), and performed similarly at day 168. At day 84, the carbon management index (CMI) was > 100% in all the organic treatments in comparison with Chem, except for WETD. At day 168, a + 30% CMI was registered in WETD and WETC> BWC> DRYD and DRYC> Chem.
Conclusion
This pattern was related to a generally marked δ13C depletion being confirmed by Δ13C, thus indicating the conservation of the carbon form compost, this very likely being related to the preferential lignin accumulation.
Novelty statement
There is increasing interest in improving soil carbon management; to this aim, the re-utilization of recycled organic waste is often claimed to be safe, successful and sustainable. However, little research has been carried out in dealing with this topic as regards food waste which is an ever-increasing source of organic matter, possibly recycled for agricultural uses. Food waste anaerobic digestates and composts can have a homogeneous 13C natural abundance signature, thus allowing the study of its fate in soil. By so doing, this study represents the first attempt to use this technique in this field. Moreover, coupled with studying the carbon management index, this study represents a first insight into the context of the rational soil carbon management in a succession of organic-mineral fertilization strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been a growing emphasis by governments in addressing greenhouse gas (GHG) emission control and soil organic carbon (SOC) conservation/storage [1,2,3]. Consequently, there is an increasing interest in strategies for managing OW which can mitigate GHG emissions and contribute to preserving or enhancing SOC stocks [4]. The anaerobic digestion of various OWs, including animal slurry, sewage sludge, and agricultural/agro-industrial residues, has emerged as a promising approach for achieving both objectives [5]. Anaerobic digestion (AD) has been recognized for its ability to reduce GHG emissions as compared to alternative processes, such as landfilling or incineration. Simultaneously, it serves as a source of renewable energy by means of biogas production, provides nutrients for crops, and offers organic carbon for soil enrichment via the use of digestates [6, 7]. In addition to the above-mentioned OWs, there has been a recent surge in the collection of food waste which, if mismanaged (e.g., incineration or landfill), poses a significant GHG impact. Hence, anaerobic digestion becomes an attractive solution for managing food waste [8, 9]. Despite its benefits, the direct application of raw anaerobic digestates to soil comes with challenges, such as ammonia (NH3) emissions and the potential release of carbon dioxide (CO2), nitrous oxide (N2O), and methane (CH4) [10,11,12,13,14,15]. To limit these issues, a common practice involves combining AD with composting to produce biologically stable products suitable for sound soil application [9]. While the nutritional aspects of anaerobic digestates and the resulting composts have been extensively studied, there is a notable research gap concerning the fate of SOC following the agricultural use of these recycled products, particularly in the case of raw and composted anaerobic digestates from food waste [16,17,18]. Given the crucial role of SOC in ecosystems and the growing interest in its conservation and potential increase, understanding the soil health status has become crucial [19]. Labile carbon, assessed through weak oxidation using potassium permanganate (KMnO4), is increasingly being considered an indicator for evaluating soil health and functionality, contributing to the calculation of the carbon management index (CMI) [20, 21]. The CMI aids in determining the relative sustainability of different management options as compared to a reference system (i.e. organic vs. chemical fertilization). In the literature, authors frequently use the CMI to analyze the changes in SOC quality resulting from various management practices [22, 23]; for example, Sodhi et al. [22] have utilized this approach to analyze SOC variations following long-term organic, mineral or combined fertilization or different soil management. In addition to labile carbon, the utilization of the 13C natural abundance (δ13C) tracer technique can provide valuable insights into carbon mineralization processes in soils [24, 25]. More specifically the biological process due to the microbial activity occurring during the anaerobic digestion and/or the composting of OW may reduce the inherent variability of their 13C signature, thus increasing the possible success of this approach [26,27,28]. In addition to this, researchers have often used the Δ13C to gain deeper understanding of the carbon mineralization process in soils [29]. This approach takes into account the fractionation processes (13C vs.12C) occurring following microbial activity, especially in the presence of a nutrient source (mainly nitrogen), such as those added with chemical fertilization in soil [30, 31]. Given that anaerobic digestates and compost are used as potential fertilizer substitutes, comparing their impact on Italian ryegrass, a nitrogen-sensitive, fast-growing species, seemed to be particularly relevant in this system [32, 33].
To investigate the effects of soil application, wet and dry-batch digestates from food waste, along with their homologous composts, were utilized as fertilizers (300 kg of available N ha− 1). This study, conducted through two consecutive growth cycles (84 + 84 days), involved a pot test on ryegrass with and without chemical nitrogen addition (180 kg ha− 1). At the conclusion of each crop cycle (days 84 and 168), cumulative plant dry biomass and N-uptake were calculated. Moreover, the pot soil was analyzed for total organic carbon and its labile fraction (KMnO4 oxidizable C) to assess the carbon management index. The δ13C and the Δ13C values were analyzed to provide a comprehensive understanding of the processes occurring in the soil and the fate of the organic carbon introduced through the compost.
Materials and methods
Organic Products
Two anaerobic digestates and two composts were compared in this study. An anaerobic digestate was collected after two weeks of thermophilic wet digestion of (100%) slurried food waste (WETD). Its homologous compost was obtained after 10 weeks of composting of the screw-pressed WETD, following the addition of green waste (25% w: w); this was called WETC. Another anaerobic digestate was collected after four weeks of mesophilic dry-batch digestion of a mixture (90% w: w) of food- and green waste (DRYD), and its homologous compost was obtained after 8 weeks of composting of DRYD with recirculation of the solid fraction as bulking agent (50% w: w); this was called DRYC. In addition to these, a bio-waste compost without the addition of anaerobic digestates (BWC) was used as an organic reference in the pot test. The pH, electrical conductivity (EC), total solids (TS) and volatile solids (VS), in addition to NH+ 4-N and NO− 3-N, and the oxygen uptake rate (OUR) [34], were assessed on products as they were. Total organic carbon (TOC) and total nitrogen (TN) were determined using an elemental analyzer on freeze-dried and ball-milled samples. The δ13C (‰) of the products tested were determined using a coupled mass spectrometer (DELTA V Advantage; Thermo Electron Germany) and expressed according to the following equation:
where R = 13C/12C. The main characteristics of the composts compared are reported in Table 1. The nutrient content, as well as the trace element, was determined using ICP after microwave assisted acid digestion (HNO3 70% + HCl 37%) on ≈ 250 mg of ball-milled sample; they are reported in Tables 2 and 3.
Pot test
A two-stage pot experiment was conducted using soil collected from the upper layer (0–20 cm) after litter removal in a field in Bologna, Italy. The soil had the following main characteristics: pH 7.90, sand content 18.4%, clay content 39.1%, silt content 42.5%, total Kjeldahl nitrogen (TKN) 0.160%, carbon-to-nitrogen ratio (C: N) 8.3, and Olsen phosphorus (Olsen-P) 5.00 mg P kg− 1. In this experiment, the four organic products (WETD, DRYD, WETC, DRYC) were applied to 1 kg of pot soil at 300 kg ha− 1 of available nitrogen considering a 30 cm layer (arable layer) and a bulk density of 1.3 kg dm− 3 (Table 1S). Furthermore, the experiment included one organic and one chemical (BWC and Chem) reference treatment at the same nitrogen rate. Two-liter pots (drilled at the bottom) pre-filled with 1 L of sand (to avoid water-logging) were filled with the amended soil and arranged in a randomized complete design with three replications.
Ryegrass was cultivated for 84 days under the following conditions: 60% water holding capacity (WHC), a 14/10-hour light/dark photoperiod, and temperatures of 23°/13°C. After the initial growth period, the soil was potted for a subsequent growth season, lasting an additional 84 days. During this second cycle, the, ryegrass received nitrogen fertilization (as NH4NO3) to ensure a nitrogen supply (180 kg ha− 1 of available N). During the experiment, tissue samples were collected three times during both the first and the second growth cycles (every 28 days). Root samples were collected on the last day of sampling (day 84 and day 168). All the harvested tissue and root samples were dried at 70 °C in a forced-air oven until a constant weight was achieved. The dry biomasses (DWs) from the various tissue harvests were summarized. The TN content in the tissue and the root was determined using an elemental analyzer (DELTA V Advantage; Thermo Electron Germany) on ball-milled samples. The N uptake was calculated by multiplying the TN by the DW. The data were analyzed using ANOVA, and the means were separated according to the Tukey test at p ≤ 0.05.
Total Organic Carbon, δ13C and KMnO4 Oxidizable Carbon in pot soil
On soil samples collected at the end of the first (day 84) and second (day 168) growth cycles, the total organic carbon content, as well as the δ13C, were determined as previously described for the compost. In addition, the KMnO4 oxidizable C (CL) content was determined on 2.5 g of pot soil according to Weil et al. [35].
The carbon management index (CMI) was obtained according to the method proposed by Blair et al. [21]:
where CPI was calculated according to the following equation:
where “C treated soil” was the organic carbon (g kg-1) from the soil treated with the organic products (WETD; WETC; DRYD; DRYC; BWC), and “C reference soil” was the organic carbon (g kg-1) in the soil from Chem.
The LI was calculated according to the following equation:
$${\text{LI}} = \left( {{\text{C}}\,{\text{lability}}\,{\text{treated}}\,{\text{soil}}} \right)/\left( {{\text{C}}\,{\text{lability}}\,{\text{reference}}\,{\text{soil}}} \right)$$(4)
Where the “C lability treated soil” was the carbon lability from the soil treated with the different organic products (WETD; WETD; DRYD; DRYC; BWC), and “C lability reference soil” was the carbon lability in the soil from Chem. The C lability was expressed as the ratio of labile C (CL) to non-labile C (CNL). Non-labile C was determined to be the difference between the total C content and the CL content of the soil. The data were analyzed using ANOVA, and the means were separated according to the Tukey test at p ≤ 0.05.
Results
Organic Products
Table 1 reports that WETD and DRYD had similar pH values, ranging from 8.4 to 8.9, also having limited variation in TS (24.8 vs. 34.0 mg g− 1), such as VS and TOC reaching approximately 55.0% and 30.0% in WETD and DRYD, respectively. Both anaerobic digestates displayed similar biological stability (OUR ≈ 60 mmol O2 kg− 1 VS h− 1). However, WETD and DRYD had contrasting N content (3.5 vs. 1.6%), resulting in significantly different C: N ratios (9 vs. 20). The composted products (WETC and DRYC) exhibited pH values between 7.3 and 10. They had different TS (63.0 vs. 76.0%) and VS (39.0 vs. 43.0%) but similar TOC (24.2 vs. 25.5%). Their N content was different (2.5 vs. 18%), leading to lower C: N ratios (10 vs. 14). Both composts (WETC and DRYC) demonstrated higher stability than the original digestates (≤ 10 mmol O2 kg− 1 VS h− 1).
The δ13C value of the AD ranged from − 22.23 to -23.32 (WETD and DRYD). Moreover, the δ13C shift after composting showed that WETC had a strongly depleted 13C signature as compared to its homologous digestate while DRYC displayed a decreasing δ13C (-26.44 and − 22.13). In this context, the reference compost (BWC) had a pH within the range of the products investigated, with higher TS and intermediate VS. As compared to the products investigated, BWC had lower TOC and N, resulting in a generally lower C: N ratio and a δ13C value of -26.28.
The nutrient content of the food waste products differed significantly, more specifically, the P and the calcium (Ca) content of WETD and DRYD varied notably, with the former ranging from ≈ 9000 to ≈ 5000 mg kg− 1 and the latter from ≈ 40,000 to ≈ 70,000 mg kg− 1 (Table 2). Other nutrients, such as potassium (K), magnesium (Mg), and sulfur (S), had narrower ranges (Table 2). In comparison to the homologous digestate, WETC showed decreasing P, Ca and S and increasing K and Mg (Table 2). While DRYC showed increasing P, Mg, and S, and decreasing K. The reference compost (BWC) was in the lower range for all the nutrient considered. According to Table 3, the trace element determined in WETD exhibited a slightly higher concentration compared to DRYD. Both composts (WETC and DRYC) showed comparable levels of trace element, being generally more concentrated in the composts than in the homologous digestates. Furthermore, BWC displayed a trace element content in the range of the food waste products under investigation. It should be noted that all the food waste products being compared met the requirements outlined in the EU fertilizer regulation [36].
Pot test
As reported in Table 4 the pot test showed that the different treatments affected the ryegrass dry biomass (P < 0.05). At the end of the first growth cycle (day 84) the DW tissue ranged from 2.76 to 4.61 g pot− 1 in both BWC and Chem respectively. In this context, WETD and WETC were intermediate (3.31 g pot− 1, on average), while DRYD and DRYC had lower averages (2.60 g pot− 1), in the same range as BWC (2.76 g pot− 1). The root had a different pattern; Chem still performed the best (2.50 g pot− 1), while all the other treatment separated poorly although DRYD and DRYC averaged 1.35 g pot− 1, in the lower range, and WETD and WETC attained 1.54 and 1.67 g pot− 1, in the same range as BWC (1.41 g pot− 1). At the end of the second growth cycle (day 168), all the other organic treatments (WETD; DRYD; WETC; DRYC) and references (BWC and Chem) averaged 1.65 g pot− 1. At the same sampling time (day 168), the root averaged 1.00 g pot− 1, regardless of the treatment. At the end of first cycle of cultivation (day 84), the N from the ryegrass tissue was the highest in Chem (193 g pot− 1). The WETD and WETC ranged lower (86 g pot− 1), while DRYD and DRYC attained 55 g pot− 1, in the same range as BWC (60 g pot− 1). At the end of the second growth cycle (day 168), the ryegrass tissue averaged 62 mg pot− 1 N uptake regardless of the treatment. The root N uptake at the end of the first cycle (day 84) was the best in Chem (29 mg pot− 1), while all the other treatments averaged 17 mg pot− 1. At the second sampling (day 168), the root N uptake was the worst in Chem (14 mg pot− 1), while DRYD and DRYC attained the best (23 mg pot− 1), the other treatments (WETD and WETC) being intermediate, in the same range as BWC (20 mg pot− 1, on average).
Total, non-labile and KMnO4 Oxidizable Carbon
Table 5 reports the SOC in the pot soil at the end of the first and second growth cycles (days 84 and 168). In this context at day 84, the end of the first cycle, Chem showed the poorest SOC (6.68 g kg− 1). Of the food waste based treatments at the same sampling time (day 84), DRYD performed the best (8.73 g kg− 1), while the other treatments (WETD; WETC; DRYC) and the organic reference (BWC) averaged at 8.00 g kg− 1. At the second sampling (day 168), Chem exhibited poor SOC (6.27 g kg− 1). At the same time, of the food waste based treatments, DRYD and DRYC attained the best SOC (7.95 g kg− 1, on average), in the same range of BWC (7.88 g kg− 1), while WETD and WETC aligned at an intermediate value (7.22 g kg− 1, on average). Table 5 also reports the CNL. At day 84, this was reported to be 6.47 g kg− 1 in Chem. Of the food waste products compared, DRYD attained the best CNL (8.46 g kg− 1), while the other treatments (WETD; WETC; DRYC) averaged 7.69 g kg− 1, in the same range as BWC (7.61 g kg− 1). At the second sampling time (day 168), the CNL in Chem performed poorly, being 6.07 g kg− 1. At the same time, of the organic treatments, DRYD and DRYC had the best CNL (7.71 g kg− 1, on average), in the same range as BWC (7.63 g kg− 1), and higher than WETD and WETC (6.95 g kg− 1, on average). Table 5 also reports CL at day 84 which was 0.209 g kg− 1 in Chem, in the low range. At the same sampling time (day 84), CL varied from 0.202 to 0.272 g kg− 1 in WETD and DRYD, while WETC, DRYC and BWC averaged intermediate (0.249 g kg− 1). At the end of the second growth cycle (day 168), Chem was 0.195 g kg− 1 (the worst). At the same sampling time, amongst the food waste based treatments, WETC showed the best CL (0.296 g kg− 1), while WETD; DRYD and DRYC averaged 0.239 g kg− 1, in the same range as BWC (0.254 g kg− 1).
Carbon pool Index (CPI), Lability (L), Lability Index (LI), and Carbon Management Index (CMI)
Table 5 also reports the CPI. At the end of the first growth cycle (day 84), it was unaffected by the treatment, averaging 1.21. On the contrary, at the end of the second growth period (day 168), DRYC was the best (1.29), in the same range as BWC (1.26), followed by DRYD (1.25), WETD and WETC (1.15, on average). Lability is also shown in Table 5; at the end of the first cycle (day 84), the data showed that this parameter was unaffected by the treatments, averaging 3.15%. At the end of the second growth cycle at day 168, WETC and DRYC performed the best and the worst (4.17 and 2.83%, respectively). The other treatments (WETD; DRYD) were intermediate (3.45%, on average), in the same range as Chem (3.23%) and BWC (3.35%). The LI reported in Table 5 showed that, at day 84, this parameter was unaffected by the treatments, averaging 0.97. At the second sampling time (day 168), WETC had the best LI (1.29), while DRYC had the worst (0.88); WETD and DRYD were intermediate (1.07, on average), in the same range as BWC (1.04). Finally, Table 5 reports the CMI. At the end of the first cycle of cultivation (day 84), this was the best in DRYD and DRYC (127.4%, on average). At the same sampling time, WETC was at a lower level (114.1%), in the same range as the organic reference BWC (118.3%), WETD was found to be the lowest (95.9%). At the second sampling time (day 168), WETC was the best (152.7%), followed by DRYD and DRYC (134.4% on average), in the same range as BWC (130.4%); WETD was found to be the lowest (120.2%).
Pot soil δ13C and Δ13C at the end of the Two Ryegrass Growth Cycles
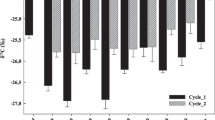
As reported in Fig. 1, at the end of the first growth cycle (day 84), the δ13C (‰) of the pot soil from Chem was − 25.40. Figure 1 also showed that, at the same sampling date, some of the treatments compared had a more depleted 13C signature (‰) in comparison to Chem: WETC (-25.96); DRYD (-26.34); DRYC (-26.02), in the same range as BWC (-26.21), while WETD was in the same range as Chem at -25.51. At the second sampling on day 168, Chem was at -25.21. In comparison to these, the food waste based treatments showed more depleted δ13C (‰): WETC (-26.02); DRYD (-26.06); DRYC (-26.32), in the same range as BWC (-26.16). Also in this case, WETD was similar to Chem at -25.31. Figure 2 reports the Δ13C(‰) of the treatments compared at the two time periods (days 84 and 168). In this context, WETD had a very similar Δ13C (‰) at the two sampling times (-0.107 and − 0.210). Of the other food waste based products, WETC showed a slight shift to a more depleted Δ13C(‰) over time (from − 0.557 to -0.817), while the Δ13C(‰) from DRYD was unaffected over time (-0.933 vs. -0.856). By contrast, DRYC had a notable Δ13C(‰) depletion (-0.611 vs. -1.111), the BWC pattern being similar, although to a lesser extent (-0.809 vs. -0.952).
– δ13C isotope natural abundance [δ13C (‰)] in the pot soil in the different treatments at the end of the two growth cycles (days 84 and 168). WETD: digestate from the wet digestion of food waste; WETC: compost from WETD; DRYD: digestate from the dry-batch digestion of food waste; DRYC: compost from DRYD; BWC: reference compost from bio-waste. Chem: chemical reference (NH4NO3 + KH2PO4). Error bars: SE n. = 3
– δ13C isotope natural abundance calculated vs.Chem [Δ13C(‰)]in the pot soil in the different organic treatments at the end of the two growth cycles (days 84 and 168). WETD: digestate from the wet digestion of food waste; WETC: compost from WETD; DRYD: digestate from the dry-batch digestion of food waste; DRYC: compost from DRYD; BWC: reference compost from bio-waste. Chem: chemical reference (NH4NO3 + KH2PO4). Error bars: SE n. = 3
Discussion
Appropriate agricultural utilization of recycled organic matter within the context of conserving and increasing organic carbon primarily depends on the quality of the organic sources utilized. In the European Union, a crucial consideration for optimal SOC management is the biological stability of recycled OW. This is widely recognized for its impact on soil organic carbon mineralization, resulting in CO2 losses, and potential emissions of other GHGs, such as N2O and CH4 [37]. According to the EU fertilizer regulation, the digestates and composts compared in this study demonstrated either unstable (WETD and DRYD) or stable (WETC and DRYC) characteristics based on the OUR threshold set at 25 mmol O2 kg− 1 VS h− 1 for sound soil utilization [36]. The varying biological stability observed aligned with the different biological processes utilized. Anaerobic digestion, especially when integrated with composting, is known to efficiently reduce the easily degradable organic matter [37,38,39]. In addition, the wet and dry processes, due to their distinct organic carbon loading capacities, require different amounts of green waste, influencing the nitrogen content and C: N ratio of digestates and composts [40,41,42]. This difference in the C: N ratio not only affected plant nutrition as proven by the pot test, but also influenced the total organic carbon applied to the soil. Plans for fertilization typically align with crop nitrogen requirements regardless of the organic matter content of the products; in this study WETD and WETC provided approximately 7 Mg ha− 1 organic carbon, while the dry-batch food waste products (DRYD and DRYC), with their higher C: N ratio, supplied higher organic carbon (≈ 10–15 Mg ha− 1). Consequently, pot soils treated with DRYD and DRYC exhibited the highest SOC at the end of both cultivation cycles. For a more in-depth insight, it therefore appeared that, in the digestate and the compost treatments following the external mineral fertilizer application, the SOC decreased by 10 and 3%, respectively. This occurred regardless of their origin, in agreement with the different stabilization level previously mentioned. However, in the pots treated with the anaerobic digestates, and even more in those treated with compost, the native soil carbon appeared to be protected from mineralization, as suggested by the δ13C which will be additionally discussed below.
As mentioned in the Introduction, in the light of improving soil health, studying labile-C and the CMI, can contribute to sustainable land management practices more than the SOC measurement [43,44,45]. Regarding the above, some information about the utilization of raw and composted sewage sludge which improves the CMI in sandy soil has been reported by Kalisz et al. [46], and some information concerning the positive role of biochar on CMI by Yang et al. [47], while van Midden et al. [48] reported the positive, and rapid, effect of the liquid fraction of a “general” anaerobic digestate on labile-C on the microbial community, thus showing the digestate solid fraction had higher long-term effect on fungi activity. The wet anaerobic digestate (WETD) tested in this study showed the lowest CMI, thus indicating a poor effect on carbon management in the context of one simulated growing season, this also being detectable in the context of two seasons. On the contrary, the composted product (WETC) had a higher CMI, also increasing over time, thus proving the efficiency of composting on this parameter. The other food waste products from dry-batch digestion (DRYD and DRYC) showed a steadier CMI, thus suggesting the possible role of a higher green waste addition. The green waste addition could have played a key role regarding this parameter since the possible effect of lignin on the KMnO4 oxidizable C and on the derived CMI has been recognized in the literature [49]. Christy et al. [50] reported the effect of a phenolic compound (such as lignin) in the assessment of KMnO4 oxidizable C, also suggesting a general increase in this fraction over time, due to its resistance to degradation and its consequent preferential accumulation. This issue has been widely debated; however, KMnO4 oxidizable C has often been recognized as reflecting practices which promote organic matter accumulation or stabilization, and can therefore be a useful indicator of long-term soil C sequestration [51]. In this context, the analysis of δ13C, and more importantly of Δ13C, can be useful for a complete understanding. It is widely acknowledged that, during the soil organic matter mineralization process, the microbial fractionation of 13C vs.12C occurs, generally leading to an enrichment of 13C and consequently less 13C depleted soil [52, 53]. Simultaneously, lignin, which is highly resistant to degradation, tends to build up preferentially over cellulose and hemicellulose, both of which are abundant in composts [28]; therefore, investigating δ13C could provide valuable insights into the differences between treatments and sampling times, including the effects of chemical nitrogen fertilization. The data presented in the present study revealed that, after both the first and the second growth cycle, the compost-treated pots were those which especially exhibited a greater depletion of δ13C as compared to the standard chemical fertilization treatment. At the same time, the dry-batch derived digestate also showed a similar trend. This was in agreement with the probable preferential build-up of lignin as a driving force for the δ13C pattern observed in the above-mentioned treated soil after the first cultivation cycle. This finding could be additionally supported by the different initial δ13C values of the food waste products tested. In fact, WETD and DRYC had very similar ranges (-22.23 and − 22.13), but resulted in significantly different pot soil δ13C values after 168 days (-25.30 and − 26.32), this also being proven by the very different Δ13C values (-0.21 and − 1.11). These outputs were consistent with the findings of Christy et al. [50]; in addition, they were also consistent with the general increase in the CMI over time. More specifically, Christy et al. [50] pointed out that labile C assessment via KMnO4 oxidation can be affected by the presence of many different organic molecules from the entire spectrum of organic matter; however, they are particularly abundant in polyphenolic compounds, such as lignin and the tannins [54].
Conclusion
The characteristics of the anaerobic digestates from food waste varied significantly depending on the processes applied. However, the raw digestates exhibited poor stabilization. The composting effectively enhanced the biological stability of the raw digestates, having minimal impact on their fertilizing capacity but promoting greater soil organic carbon conservation. In addition, not only was the total soil organic carbon affected, its quality was also affected. When compared to chemical fertilization, all the organic treatments (except for WETD) had a higher carbon management index. This index also increased over time, following chemical fertilization. This trend corresponded to a substantial depletion of the soil δ13C, thus indicating the conservation of the organic carbon applied with the food waste products. This trend was ascribed to the preferential accumulation of lignin, thus suggesting that waste management processes incorporating higher quantities of green waste could significantly contribute to increasing soil organic carbon and its quality within a succession of organic-chemical fertilization practices.
Data Availability
Enquiries regarding data availability should be directed to the authors.
References
Fawzy, S., Osman, A.I., Doran, J., Rooney, D.W.: Strategies for mitigation of climate change: A review, (2020). https://doi.org/10.1007/s10311-020-01059-w
Francaviglia, R., Almagro, M., Vicente-Vicente, J.L.: Conservation agriculture and soil organic carbon: Principles, Processes, Practices and Policy Options, (2023). https://doi.org/10.3390/soilsystems7010017
Wiesmeier, M., Urbanski, L., Hobley, E., Lang, B., von Lützow, M., Marin-Spiotta, E., van Wesemael, B., Rabot, E., Ließ, M., Garcia-Franco, N., Wollschläger, U., Vogel, H.J., Kögel-Knabner, I.: Soil organic carbon storage as a key function of soils - a review of drivers and indicators at various scales, (2019). https://doi.org/10.1016/j.geoderma.2018.07.026
Uen, T.S., Rodríguez, L.F.: An integrated approach for sustainable food waste management towards renewable resource production and GHG reduction. J. Clean. Prod. 412 (2023). https://doi.org/10.1016/j.jclepro.2023.137251
Jimenez, J., Grigatti, M., Boanini, E., Patureau, D., Bernet, N.: The impact of biogas digestate typology on nutrient recovery for plant growth: Accessibility indicators for first fertilization prediction. Waste Manage. 117 (2020). https://doi.org/10.1016/j.wasman.2020.07.052
Chen, Y., Pinegar, L., Immonen, J., Powell, K.M.: Conversion of food waste to renewable energy: A techno-economic and environmental assessment. J. Clean. Prod. 385 (2023). https://doi.org/10.1016/j.jclepro.2022.135741
Tonini, D., Albizzati, P.F., Astrup, T.F.: Environmental impacts of food waste: Learnings and challenges from a case study on UK. Waste Manage. 76 (2018). https://doi.org/10.1016/j.wasman.2018.03.032
European Commission: Directive 2008 / 98 / EC on Waste. Waste Framework Directive (2008)
van der Linden, A., Reichel, A.: Bio-waste in Europe: Turning challenges into opportunities, (2020)
Herrera, A., D’Imporzano, G., Zilio, M., Pigoli, A., Rizzi, B., Meers, E., Schouman, O., Schepis, M., Barone, F., Giordano, A., Adani, F.: Environmental performance in the production and use of recovered fertilizers from Organic Wastes treated by anaerobic digestion vs synthetic Mineral fertilizers. ACS Sustain. Chem. Eng. 10 (2022). https://doi.org/10.1021/acssuschemeng.1c07028
Willén, A., Jönsson, H., Pell, M., Rodhe, L.: Emissions of Nitrous Oxide, methane and Ammonia after Field Application of Digested and Dewatered Sewage Sludge with or without addition of Urea. Waste Biomass Valorization. 7 (2016). https://doi.org/10.1007/s12649-015-9456-2
Møller, J., Boldrin, A., Christensen, T.H.: Anaerobic digestion and digestate use: Accounting of greenhouse gases and global warming contribution, (2009). https://doi.org/10.1177/0734242X09344876
Walling, E., Vaneeckhaute, C.: Greenhouse gas emissions from inorganic and organic fertilizer production and use: A review of emission factors and their variability, (2020). https://doi.org/10.1016/j.jenvman.2020.111211
Bernal, M.P., Alburquerque, J.A., Moral, R.: Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour Technol. 100 (2009). https://doi.org/10.1016/j.biortech.2008.11.027
Zilio, M., Pigoli, A., Rizzi, B., Herrera, A., Tambone, F., Geromel, G., Meers, E., Schoumans, O., Giordano, A., Adani, F.: Using highly stabilized digestate and digestate-derived ammonium sulphate to replace synthetic fertilizers: The effects on soil, environment, and crop production. Sci. Total Environ. 815 (2022). https://doi.org/10.1016/j.scitotenv.2022.152919
Gunnarsson, A., Bengtsson, F., Caspersen, S.: Use efficiency of nitrogen from biodigested plant material by ryegrass. J. Plant Nutr. Soil Sci. 173 (2010). https://doi.org/10.1002/jpln.200800250
Grigatti, M., Cavani, L., Marzadori, C., Ciavatta, C.: Recycling of dry-batch digestate as amendment: Soil C and N dynamics and ryegrass nitrogen utilization efficiency. Waste Biomass Valorization. 5 (2014). https://doi.org/10.1007/s12649-014-9302-y
Czekała, W., Nowak, M., Piechota, G.: Sustainable management and recycling of anaerobic digestate solid fraction by composting: A review, (2023). https://doi.org/10.1016/j.biortech.2023.128813
Bonfante, A., Basile, A., Bouma, J.: Targeting the soil quality and soil health concepts when aiming for the United Nations Sustainable Development Goals and the EU Green Deal. SOIL. 6 (2020). https://doi.org/10.5194/soil-6-453-2020
Bongiorno, G., Bünemann, E.K., Oguejiofor, C.U., Meier, J., Gort, G., Comans, R., Mäder, P., Brussaard, L., de Goede, R.: Sensitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 99 (2019). https://doi.org/10.1016/j.ecolind.2018.12.008
Blair, G.J., Lefroy, R.D., Lisle, L.: Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust J. Agric. Res. 46 (1995). https://doi.org/10.1071/AR9951459
Sodhi, G.P.S., Beri, V., Benbi, D.K.: Using carbon management index to assess the impact of compost application on changes in soil carbon after ten years of rice-wheat cropping. Commun. Soil. Sci. Plant. Anal. 40 (2009). https://doi.org/10.1080/00103620903326024
Mandal, U.K., Yadav, S.K., Sharma, K.L., Ramesh, V., Venkanna, K.: Estimating permanganate-oxidizable active carbon as quick indicator for assessing soil quality under different land-use system of rainfed alfisols. Indian J. Agric. Sci. 81, (2011)
Inácio, C.T., Magalhães, A.M.T., Souza, P.O., Chalk, P.M., Urquiaga, S.: The relative isotopic abundance (δ13C, δ15N) during composting of agricultural wastes in relation to compost quality and feedstock. Isot. Environ. Health Stud. 54 (2018). https://doi.org/10.1080/10256016.2017.1377196
Glaser, B., Bol, R., Preedy, N., McTiernan, K.B., Clark, M., Amelung, W.: Short-term sequestration of slurry-derived carbon and nitrogen in temperate grassland soil as assessed by 13C and 15N natural abundance measurements. J. Plant Nutr. Soil Sci. 164 (2001). https://doi.org/10.1002/1522-2624(200110)164:5<467::AID-JPLN467>3.0.CO;2-Q
Menichetti, L., Houot, S., van Oort, F., Kätterer, T., Christensen, B.T., Chenu, C., Barré, P., Vasilyeva, N.A., Ekblad, A.: Increase in soil stable carbon isotope ratio relates to loss of organic carbon: Results from five long-term bare fallow experiments. Oecologia. 177 (2015). https://doi.org/10.1007/s00442-014-3114-4
Volk, M., Bassin, S., Lehmann, M.F., Johnson, M.G., Andersen, C.P.: 13C isotopic signature and C concentration of soil density fractions illustrate reduced C allocation to subalpine grassland soil under high atmospheric N deposition. Soil. Biol. Biochem. 125 (2018). https://doi.org/10.1016/j.soilbio.2018.07.014
Lynch, D.H., Voroney, R.P., Warman, P.R.: Use of 13C and 15N natural abundance techniques to characterize carbon and nitrogen dynamics in composting and in compost-amended soils. Soil. Biol. Biochem. 38 (2006). https://doi.org/10.1016/j.soilbio.2005.04.022
Wang, G., Jia, Y., Li, W.: Effects of environmental and biotic factors on carbon isotopic fractionation during decomposition of soil organic matter. Sci. Rep. 5 (2015). https://doi.org/10.1038/srep11043
Boström, B., Comstedt, D., Ekblad, A.: Isotope fractionation and 13C enrichment in soil profiles during the decomposition of soil organic matter. Oecologia. 153 (2007). https://doi.org/10.1007/s00442-007-0700-8
Dou, X., He, P., Cheng, X., Zhou, W.: Long-term fertilization alters chemically-separated soil organic carbon pools: Based on stable C isotope analyses. Sci. Rep. 6 (2016). https://doi.org/10.1038/srep19061
Perdigão, A., Marques, F., Pereira, J.L.S.: Effect of different Tannery Sludge composts on the production of Ryegrass: A Pot experiment. Open. Agric. J. 16 (2022). https://doi.org/10.2174/18743315-v16-e2207270
Grigatti, M., Boanini, E., Bolzonella, D., Sciubba, L., Mancarella, S., Ciavatta, C., Marzadori, C.: Organic wastes as alternative sources of phosphorus for plant nutrition in a calcareous soil. Waste Manage. 93 (2019). https://doi.org/10.1016/j.wasman.2019.05.028
Grigatti, M., Pérez, M.D., Blok, W.J., Ciavatta, C., Veeken, A.: A standardized method for the determination of the intrinsic carbon and nitrogen mineralization capacity of natural organic matter sources. Soil. Biol. Biochem. 39 (2007). https://doi.org/10.1016/j.soilbio.2006.12.035
Weil, R.R., Islam, K.R., Stine, M.A., Gruver, J.B., Samson-Liebig, S.E.: Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 18 (2003). https://doi.org/10.1079/AJAA2003003
EC: Regulation (EU) 2019/1009 Fertilizer Products. Official Journal of the European Union: (2019) (2019)
Al-Rumaihi, A., McKay, G., Mackey, H.R., Al-Ansari, T.: Environmental impact assessment of food waste management using two composting techniques. Sustain. (Switzerland). 12 (2020). https://doi.org/10.3390/su12041595
Wilkie, A.C.: Anaerobic Digestion: Biology and Benefits. Dairy Manure Management: Treatment, Handling, and Community Relations. (2005)
Kraemer, T., Gamble, S.: Integrating Anaerob. Digestion Composting Biocycle 55, (2014)
Meegoda, J.N., Li, B., Patel, K., Wang, L.B.: A review of the processes, parameters, and optimization of anaerobic digestion, (2018). https://doi.org/10.3390/ijerph15102224
Nkuna, R., Roopnarain, A., Rashama, C., Adeleke, R.: Insights into organic loading rates of anaerobic digestion for biogas production: A review, (2022). https://doi.org/10.1080/07388551.2021.1942778
Rico, C., Montes, J.A., Lobo, A.: Dry batch anaerobic digestion of food waste in a box-type reactor system: Inoculum preparation and reactor performance. J. Clean. Prod. 251 (2020). https://doi.org/10.1016/j.jclepro.2019.119751
Culman, S., Freeman, M., Snapp, S.: Procedure for the Determination of Permanganate Oxidizable Carbon. KBS POXC Protocol – Last revised. 1, 1–5 (2012)
Vieira, F.C.B., Bayer, C., Zanatta, J.A., Dieckow, J., Mielniczuk, J., He, Z.L.: Carbon management index based on physical fractionation of soil organic matter in an Acrisol under long-term no-till cropping systems. Soil. Tillage Res. 96 (2007). https://doi.org/10.1016/j.still.2007.06.007
Zhao, F., Yang, G., Han, X., Feng, Y., Ren, G.: Stratification of carbon fractions and carbon management index in deep soil affected by the grain-to-Green Program in China. PLoS One. 9 (2014). https://doi.org/10.1371/journal.pone.0099657
Kalisz, B., Łachacz, A., Głażewski, R., Grabowski, K.: Labile organic carbon fractions after amendment of sandy soil with municipal sewage sludge and compost. J. Elem. 22 (2017). https://doi.org/10.5601/jelem.2017.22.1.1264
Yang, X., Wang, D., Lan, Y., Meng, J., Jiang, L., Sun, Q., Cao, D., Sun, Y., Chen, W.: Labile organic carbon fractions and carbon pool management index in a 3-year field study with biochar amendment. J. Soils Sediments. 18, 1569–1578 (2018). https://doi.org/10.1007/s11368-017-1874-2
van Midden, C., Harris, J., Shaw, L., Sizmur, T., Pawlett, M.: The impact of anaerobic digestate on soil life: A review, (2023). https://doi.org/10.1016/j.apsoil.2023.105066
Skjemstad, J.O., Swift, R.S., McGowan, J.A.: Comparison of the particulate organic carbon and permanganate oxidation methods for estimating labile soil organic carbon. Aust. J. Soil Res. 44, 255–263 (2006). https://doi.org/10.1071/SR05124
Christy, I., Moore, A., Myrold, D., Kleber, M.: A mechanistic inquiry into the applicability of permanganate oxidizable carbon as a soil health indicator. Soil Sci. Soc. Am. J. 87 (2023). https://doi.org/10.1002/saj2.20569
Hurisso, T.T., Culman, S.W., Horwath, W.R., Wade, J., Cass, D., Beniston, J.W., Bowles, T.M., Grandy, A.S., Franzluebbers, A.J., Schipanski, M.E., Lucas, S.T., Ugarte, C.M.: Comparison of Permanganate-Oxidizable Carbon and Mineralizable Carbon for Assessment of Organic Matter Stabilization and mineralization. Soil Sci. Soc. Am. J. 80 (2016). https://doi.org/10.2136/sssaj2016.04.0106
Wynn, J.G.: Carbon isotope fractionation during decomposition of organic matter in soils and paleosols: Implications for paleoecological interpretations of paleosols. Palaeogeogr Palaeoclimatol Palaeoecol. 251 (2007). https://doi.org/10.1016/j.palaeo.2007.04.009
Gerzabek, M.H.: Soil organic matter (SOM) dynamics determined by stable isotope techniques. Mitteilungen Der Deutschen Bodenkundlichen Gesellschaft 87, (1998)
Crow, S.E., Lajtha, K., Filley, T.R., Swanston, C.W., Bowden, R.D., Caldwell, B.A.: Sources of plant-derived carbon and stability of organic matter in soil: Implications for global change. Glob Chang. Biol. 15 (2009). https://doi.org/10.1111/j.1365-2486.2009.01850.x
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grigatti, M. The Impact of Raw and Composted Food Waste Anaerobic Digestates on Soil Organic Carbon Management: A Pot Study. Waste Biomass Valor 15, 4915–4925 (2024). https://doi.org/10.1007/s12649-024-02501-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-024-02501-8