Abstract
Surplus availability of rice straw (RS) presents it as a potential feedstock for ethanol production. Steam explosion (SE) is considered as a green approach to extract fermentable sugars at lower cost. The present study deals with the reaction condition optimization for water and dilute acid assisted steam explosion of rice straw at different temperatures and explores the effect of structural properties of solid residue on enzymatic hydrolysis along with mass balance. SE conditions were optimized at pilot scale, raising the temperature from 170 to 200 °C in water assisted SE resulting in an increased glucan conversion from 21.4 to 42.5% at 15% solid loading using 1.5 FPU of cellulases g–1 biomass. Further, it was improved up to 58.7% by increasing the enzyme dosage to 5 FPU, although it might lead to enhanced enzyme cost by threefold. To reduce costs, small amount of dilute acid (DA) was added during SE and lowering of enzyme consumption i.e. 1.5 FPU/g cellulose has been used to achieve 65.5% glucan conversion. Varying temperature and incorporate dilute acid during pretreatment induced structural alterations in biomass evident by compositional analysis, FT-IR and mass balance. Mass balance study revealed that the overall sugar recovery i.e. 58.7 and 38.8% and theoretical yield of ethanol shall be 222 and 186 L ton–1 RS respectively, with and without DA addition.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Additon of small amount of dilute acid during steam explosion pretreatment is highly desired for enhanced sugar recovery and better process economics. The envisaged ethanol yield from the dilute acid assisted process is about 1.2 fold higher than the convential process.
Introduction
Emergent world’s fuel demand with global warming tempted to find an alternative to fossil material, which can be produced locally and biologically and thus can help to improve socio-economic upliftment of the rural sector. Therefore, it is imperative to develop and utilize renewable energy like wind, solar, geothermal, hydropower and biomass as one of the promising energy source for a more sustainable society [1,2,3]. This has even more relevance for countries that are heavily dependent on imported crude, such as India.
Biomass is the largest and surplus source of energy being used to generate heat and power over the last few decades. However, use of biomass in the form of transportation fuel has not been explored much. Presently, there are several countries for instance; the United States, the European Union, Brazil, China, Canada and India etc. have promoted the fuel ethanol production [4, 5]. Fuel ethanol in these countries has been produced using food based material like corn, sugarcane, sugar beet etc. leading to fuel vis-à-vis food debate. Therefore, lignocelluloses biomass like rice straw, cotton straw, wheat straw, sugarcane bagasse, corn stover, Acacia magnesia, etc. contains holocellulose and it could be an option to produce fuel ethanol for mitigating the fossil fuel requirement to some extent [6,7,8].
Abundance availability across the globe comprising more than 50–55% fermentable sugars present in rice straw shows a vast potential feedstock for ethanol production [9, 10]. Globally, annual rice production is 700–800 million tons. India is the second-largest producer after China, which, produce ⁓112 and 140 million tons of milled rice and paddy straw yearly [11]. In India, nearly 50% of rice straw is burnt because farmers has a very limited time about 3–4 weeks to prepare their land for next crop and therefore burning is the fastest and the cheapest way of removing waste from their land [12]. Increasing the world pollution and health problem are observed due to increase in greenhouse gases due to fuel burning activities [13]. Furthermore, rice straw does not make a suitable to use it as an animal fodder as it has large amount of silica and a very small amount of protein content. Thus, bio-processing of biomass for bio-ethanol production not just give the resolution for it managing the land as well as correspondingly lesser pollution by eluding burning, encouraging a natural source of renewable energy would result in enhancing social and economic level of rural society. Although, biomass is very rich in fermentable sugars, however, extracting of these sugars from the biomass make difficult because of recalcitrant nature of biomass. Hence, the pretreatment is prerequisite process to improve sugar recovery in biomass processing leading rupture the LCC (lignin-carbohydrate complex) structure. This polysaccharide thus escalates the cellulose accessibility towards cellulases in enzymatic saccharification. Several pretreatment techniques such as, grinding, wet milling, dilute acid, alkaline, ionic liquid, etc., are obtainable for manufacturing lignocellulosic ethanol. All aforementioned methods are expensive, energy intensive or involve the use of high bio-catalyst concentration leading to higher effluent treatment costs and thereby requiring exotic metallurgy for reactors and vessels [14,15,16,17].
Steam explosion has been explored as one of the potent, greener, and economical methods to extract and employ the carbohydrate available in biomass for industrial purpose. SE involves the compression and decompressions of biomass at high steam pressure and temperature, where, water enter inside to the biomass, moisten cellulose and dissolve hemicellulose as well slightly detach/ or displacement of lignin [8]. Dilute acid catalyzed steam explosion can effectively solubilize the xylan and considering as a potential path for lignocellulosic ethanol production. The proton (H+) ion of dilute acid (H2SO4) during SE is proficient to penetrate the rice straw cell wall resulting to cleavage the hemicelluloses β-(1→4)-glycosidic bonds and stimulate the defibrillation of RS. Moreover, the efficiency of glucan hydrolysis was firmly dealt with the structural intricacy of pretreated residue such as crystallinity, hemicellulose and lignin content along with cellulase loading, pH, temperature and required reaction conditions [18].
Present study deals with a systematic investigation on pretreatment of rice straw at varying reaction temperature performed in a continued steam explosion pilot plant of 240 kg day–1 capacity with vertical steam digester reactor (15 L capacity) at Indian Oil Corporation Limited (IOCL), R&D Centre, Faridabad, India. The objective of this research including: (a) optimization RS pretreatment condition (b) evaluating the impact of water soaking followed by supplementing/mixing dilute sulfuric acid in enhancing enzymatic hydrolysis, (c) understanding the trade-off between chemical and bio-chemical usages for cost reduction (d) interrelationship between the enzymatic hydrolysis with the physicochemical characteristics of pretreated residue to establish their relationship with enzyme saccharification. Moreover, the impact of inhibitors like acetic acid, furfural, and HMF and oligomers including gluco- and xylo-oligomers formed during SE has been evaluated on the enzymatic hydrolysis.
Materials and Methods
The collection of rice straw was conducted at the time of harvesting in the fall of year 2020 from Mohna village (28.22° N, 77.44° E), Faridabad, Haryana (North India) and milled using a knife mill with a ∼10 mm screen size and stored in an airtight polybags. The chemical/materials were an analytical grade and utilized as such with no purification. The Cellic Ctec3 enzyme (cellulases) was obtained from Praj Industries, Pune, India as a gift sample.
Steam Explosion of Rice Straw
10 kg rice straw having moisture contain of 9.0 wt% was primarily soaked/immersed in the water with a ratio of 1:20 biomass-water (w/w) for 1 h at ambient temperature. Further, the soaked rice straw was dewatered using a hydraulic press machine at 100 bar for 10 min and the resultant content having ∼60–65% moisture. Beforehand, pretreatment vertical reactor was preheated using a steam jacket for 0.5 h to reach the desired set temperature. In SE, the hopper was feed by RS at 10 kg h–1 rate to maintain a static flow towards the vertical reactor. The reaction mixture was warm up by directly steam injected in vertical reactor at different reaction temperatures ranging from 170 to 220 °C for a constant retention time of 5 min. Experiments were also conducted under acid addition in soaked rice straw. For instance, 1.1 wt% dilute sulfuric acid was sprayed on soaked and squeezed biomass and mixed thoroughly by hopper agitator at 100 rpm for 30 min followed by steam explosion pretreatment at 190 and 200 °C for 5 min. As the reaction was accomplished, the instant explosive depressurization was occurred due to unlock the ball valve and final treated slurry was obtained and collected in the slurry tank through cyclone separator. The moisture content, insoluble solid and composition of the pretreated residue were determined under prescribed condition according to NREL protocol [14]. Whereas, monomeric and oligomeric sugars and inhibitor concentrations present in pretreated hydrolysate were analyzed by the method given in Semwal et al. [15]. The SE treated rice-straw slurry was kept in a closed container and stored at 4 °C, until further use.
Enzymatic Saccharification
The pretreated slurry was separated into two parts i.e., (i) washed (by water) and (ii) unwashed (pretreated slurry as such). The unwashed SE treated RS was maintained to a pH of 5.0–5.2 by the addition of the 1N sodium hydroxide (aqueous). Enzymatic hydrolysis of pretreated samples was executed in a rotary incubator using sodium citrate buffer (0.05 M) at 50 °C using 15% WIS (water-insoluble solid) loading. 15 g pretreated residue (dry basis) from each experiment was transferred into a 500 mL Erlenmeyer flasks and mixed with sodium citrate buffer (pH 5.0) to maintain a final volume (100 ml) of reaction mixture. The slurry was pre-incubated at 50 °C for 10 min followed by adding cellulases, i.e., range from 1.5 to 5 FPU g–1 WIS. The ⁓100 µL supernatant was withdrawn from the reaction mixture at different time interval for measuring the sugar concentration through HPLC.
FT-IR
FT-IR spectrometer (FT-IR Prestige-21 Shimadzu) was utilized to find out the structural alteration of the samples and drawn the IR spectra in the range of 400–4000 cm–1 with resolution of 4 cm–1 and 200 scan per sample in an absorbance mode. The samples were prepared in drift mode with KBr in the ratio of 1:1000, ww–1 by crushing followed by pressing into tablet. Avicel PH 101 (microcrystalline cellulose) was taken as a standard sample. The zero baseline correction was improved by 10 points smoothing using KubelkaMunk model.
Process Material Balance
The process material balance was evaluated on biomass (dry basis) received pre and post steam explosion pretreatment (PT) and enzymatic hydrolysis (EH) for the targeted product of ethanol. The overall sugar recoveries after PT and EH was calculated based on the formula given in Sharma et al. [19]. Whereas, calculation of expected ethanol yield after fermentation and electricity generation (theoretical) through residual lignin (byproduct) were given in Soam et al. [20].
Results and Discussion
Selection of Reaction Condition and Approach towards Improving Sugar Conversion
Rice straw (water soaked) was performed in steam explosion digester reactor at a wide range of operating temperature from 170 to 220 °C with water/steam for 5 min residence time. The pretreatment efficiency was measured by the enzymatic hydrolysis (15% solid loading and 1.5 FPU of cellulase g–1 WIS) and it was found that the highest glucan conversion was in 200 °C (42.5%) and lowest in 170 °C (21.4%) and the detailed descriptions of glucan conversion are given in Sect. 3.4. To achieve a target of approx. 70% glucose hydrolysis, the experiment was further conducted at higher enzyme dosage from 1.5 to 5 FPU g–1 WIS. The maximum glucan conversion, i.e. 57.6% was obtained in 5 FPU of cellulase g–1 WIS at 15% solid loading. Since, by increasing the enzyme dosages improved the conversion of glucan from 42.5 to 57.6% however; it did not provide the desired result. Therefore, the addition of a small amount sulfuric acid (1.1 wt%) were attempted to the improve efficiency of steam explosion pretreatment and enhanced the glucan conversion up to ~ 22–24%. The addition of a small amount of sulfuric acid is a key strategy to enhance the sugar conversion rate of lignocellulosic biomass by facilitating the hydrolysis of complex carbohydrate structures. Sulfuric acid functions as a potent acid catalyst, donating protons (H+) that initiate the cleavage of glycosidic bonds within cellulose and hemicellulose components. This protonation triggers the depolymerisation of polysaccharides into soluble sugars, primarily glucose and xylose. Additionally, the presence of sulfuric acid establishes an acidic environment that promotes the disruption of lignin-carbohydrate linkages, further aiding in the liberation of sugars [21]. This dual mechanism of acid-catalyzed hydrolysis and lignin modification synergistically enhances the accessibility of sugars for subsequent enzymatic or chemical conversion processes, leading to improved sugar yields and overall conversion efficiency of lignocellulosic biomass [22].
Components of Native and Pretreated Residue
Table 1 summarizes the compositional analysis of untreated and treated RS along with water-insoluble solids (WIS) of respective SE treated residues. W1-170, W2-180, W3-190, W4-200, W5-210, W6-220, SA1-190, and SA2-200 refer to rice straw pretreated with water (W) or sulfuric acid (SA) from 170 to 220 °C for 5 min respectively. The increase in pretreatment severity either by increasing the temperature or by increasing the acidity of media results in lower WIS suggesting removal of non-structural sugars, extractives and solubilization of hemicelluloses. For instance, an increase in temperature from 170 to 220 °C while using water/steam as a reaction medium results in the reduction of WIS from 87.0 to 76.4%, whereas, addition of acid as a catalyst in the SE, i.e., SA1-190 and SA2-200 resulted in WIS to 71.0 and 61.0% respectively. The correlation between WIS and residual hemicellulose content in the pretreated residue was also reported by Gaur et al. [23].
Table 1 represent the glucan content in native RS, i.e., 37.8% and which increases after SE, i.e., 41.1, 42.4, 44.9, 52.0, 53.1% in W1-170, W2-180, W3-190, W4-200, W5-210 and then slightly reduced to 50.7% in W6-220. The glucan content further increased to 55.0 and 54.5% as the severity of reaction condition was increased by addition of dilute acid during SE (SA1-190 and SA2-200) indicating xylan and /or lignin removal or solubilization. Enrichment of glucan content in pretreated residue was also observed previously [19, 24].
It was also observed that the xylan content was increased from 18.3% (native RS) to 21.0 (W1-170), 20.5 (W2-180) and 20.1% (W3-190) respectively. However, the xylan content significantly dropped to 18.2 (W4-200), 13.6 (W5-210), and 11.3% (W6-220), when the pretreatment temperature raised from 200 to 220 °C and further reduced to 9.6% (SA1-190) and 8.6% (SA2-200), by addition of dilute acid during SE indicating increased xylan solubilization. It is further supported by residual acetic acid, which significantly decreased from 1.0 to 0.0% with increasing severity in W1-170 to 0.0% in SA2-200. This finding suggests that a higher pretreatment temperature and a lower pH encourage the hydrolysis of the ester linkage among acetyl and xylan, resulting in xylan solubilization during pretreatment and also more affected cellulose with a greater potential for its hydrolysis. Additionally, it was shown that the removal of xylan could increase the cell wall matrix porosity and build up nano-scale gaps (voids) between delaminated microfibril sheets, increasing the cellulose's susceptibility to cellulase [22]. It has also been previously demonstrated that enzymatic hydrolysis and xylan removal are positively correlated [25]. However, the partial degradation and generation of xylose and furfural, respectively, in the pretreatment hydrolysate that was accumulated under more severe reaction conditions likely contributes to the reduction of xylan content [26]. In pretreated residue, it is believed that the acetyl groups and lignin could prevent the cellulase from attacking the cellulose [27, 28]. Greater lignin content is produced when pretreatment harshness is enhanced during SE either via raising the temperature or increasing the acidity that can be attributed to the development of pseudo-lignin. For instance, in water assisted SE (W6-220) and dilute acid-assisted SE (SA2-200), the lignin concentration rose from 12.9% (native RS) to 23.6 and 22.2%, respectively. Similar findings of lignin content increasing with severity were previously reported [29, 30].
Analysis of Components Present in SE Treated Hydrolysate
The monomeric and oligomeric sugars in pretreatment hydrolysate are shown in Table 2, along with their breakdown products such as HMF, furfural, acetic acid, and formic acid. The sugar concentration (C6 and C5 sugar) in pretreatment hydrolysate was increased either by increasing the pretreatment temperature or by addition of acid. In water-assisted SE, sugars are mainly present in oligomeric form and these sugars reduce with raising the process temperature. It was noted that the W4-200 found maximum oligomer formation, i.e., 32.1 g L–1 whereas, SE conducted at a lower temperature (W1-170, W2-180, and W3-190) gives lower oligomer formation, i.e., 8.8, 7.5, and 7.4 g L–1 respectively, this may be attributed to the insufficient hydrolysis of glucan and xylan.
In W5-210 and W6-220, the formation of oligomers was reduced to 26.7 and 12.5 g L−1 after 200 °C due to the higher degree of hydrolysis caused by the higher temperature used to convert the oligomer to monomers. During dilute acid assisted SE, 17.2 g L–1 and 22.75 g L–1 sugars (C6 + C5) were formed in SA1-190 and SA2-200 caused by the degradation of hemicellulose to xylose and a minor quantity of glucan to glucose. HMF and furfural are produced from the further breakdown of these sugars, which are respectively formed from xylose and glucose. However, oligomers were not detected in acid-assisted SE hydrolysate indicating that the dilute acid could effectively solubilize the oligosaccharides to xylose during pretreatment supported by the reduced residual xylan content, i.e. 9.6 and 8.6% present in SA1-190 and SA2-200 respectively.
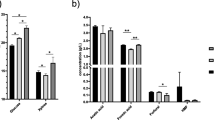
It is noticed that at lower pretreatment temperatures, such as W1-170, W2-180, and W3-190, inhibitor concentrations of HMF, furfural, acetic acid, and formic acid were found to be negligible. While adding dilute acid (SA1-190 and SA2-200) and raising the temperature from 200 to 220 °C (W4-200, W5-210, and W6-220) significantly increased the formation of inhibitor concentration, i.e. from 1.19 to 4.19 g L–1. However, the maximum inhibitor concentration was found in W6-220 (4.19 g L–1) followed by SA2-200 (2.32 g L–1) due to the higher severity in W6-220 than SA2-200. This may be argued that the formation of inhibitors is directly proportional to the severity applied during pretreatment. The degradation products formed during pretreatment can cause inhibitory effect on the growth of fermenting yeast and thereby reduce ethanol productivity. However, these degradation products formed during pretreatment are lesser than reported data (⁓1.5–2.0 g L–1). Some authors reported previously about the formation of inhibitors during SE pretreatment at higher severity [19, 31, 32].
Glucan Hydrolysis and Balance Between Chemical and Biological Catalyst
Efficiency of pretreatment and glucan hydrolysis is a significant part to the bio-ethanol conversion from lignocellulosic biomass. The pretreatment is not only solubilize xylose by destructing the biomass cell wall and dislocating the lignin but also boost the rate of enzymatic hydrolysis by increasing the accessibility of cellulase towards biomass for formation of fermentable sugars [33]. Figure 1 depicts the time course of glucan hydrolysis of SE treated RS under various reaction conditions with 15% solid loading using 1.5 FPU cellulases g–1 of WIS. It can be seen that the glucan conversion was significantly increased from 12.6 to 65.4% with raising the hydrolysis time from 24 to 72 h, however, the rate of enzymatic hydrolysis was increase in high severity (pretreatment temperature: ≤ 200 °C and acid addition) and reflected on the beginning of saccharification itself (Fig. 1). This can be explained by various factors that influence the enzymatic hydrolysis, for examples, substrate product ratio, product and enzyme inhibition and the crystallinity of cellulose etc. As the temperature of the pretreatment increases from 170 to 200 °C (W1-170 to W4-200), glucan conversion of the biomass increased from 21.4 to 42.5% at 72 h of hydrolysis. It indicated that high temperature can promote the brakeage of hydrogen bond within the crystalline area of cellulose thereby increasing the amorphous region of cellulose present in SE residue leading to enhanced glucan hydrolysis. However, upon further increasing the temperature to 210 and 220 °C (W5-210, W6-220), it decreases. The highest concentration of inhibitory products (furfural, HMF, and acetic acid) available in pretreatment hydrolysate may be the reason to inhibit cellulase and subsequently slowdown the enzymatic hydrolysis (Table 2).
Additionally, the formation of pseudo-lignin in W6-220 may be the result of condensation of sugars degradation products like HMF and furfural within or with lignin and may be responsible for lowering enzymatic hydrolysis. The pseudo-lignin acts as a physical barrier or forms a non-productive binding with the enzyme protein, impeding cellulases access to the cellulose. Furthermore, the inclusion of sulfuric acid during in the steam explosion boosted the glucan conversion in SA1-190 and SA2-200 to 57.0 and 65.4%, respectively. This might be explained by the fact that when more xylan is solubilized, the glucan becomes more susceptible to enzymatic attack. The acid catalyst during pretreatment enable the breakdown of β-1,4-glycosidic bonds by transmitting protons and protonation the glycosidic oxygen connection with the sugar monosaccharide unit, whereas the ester bond in hemicellulose is hydrolyzed by the acid through protonation of glycosidic bonds. Therefore, removing xylan makes biomass more porous, which improves water diffusion inside the channels of the pretreated residue and changes the biomass's physical properties [34].
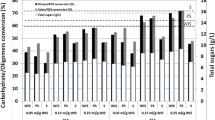
Figure 2 shows the correlation between glucan conversion and total sugar concentration during enzymatic hydrolysis of pretreated residue with xylan solubilization during the steam explosion. The order for xylan solubilization was W1-170 (0.2%) < W2-180 (4.3%) < W3-190 (5.9%) < W4-200 (21.4%) < W5-210 (42.3%) < W6-220 (52.8%) < SA1-190 (62.8%) < SA2-200 (71.3%), whereas, glucan conversion follows the order as: W1-170 (21.4%) < W6-220 (21.6%) < W2-180 (26.3%) < W3-190 (33.7%) < W5-210 (37.1%) < W4-200 (42.5%) < SA1-190 (57.0%) < SA2-200 (65.4%). In general, the xylan solubilization increases with an increase in the severity resulting to an increase in the subsequent enzymatic hydrolysis as reported by Sui et al. [35]. The maximum xylan solubilization, i.e. 71.3% was found in SA2-200 showing the maximum glucan conversion, i.e. 65.4%, which is the apparent reason for improved enzymatic accessibility. The higher solubilization of xylan in acid assisted SE might be due to labile nature of xylan towards acid hydrolysis [31]. Moreover, the close difference in severity between W6-220 and SA2-200 and W5-210 and SA1-190 with different glucan conversion, i.e. 21.6 and 65.4; 37.1 and 57.0% respectively support the labile nature of xylan towards acid during SE. Contrary to above, the results demonstrated that the pretreated residue obtained in W5-210 and W6-220 despite having the higher xylan solubilization resulted in a lower saccharification yield than W4-200. The decrease in glucan conversion and total sugar conversion in W5-210 and W6-220 can be attributed to the intensified severity of the steam explosion process. At higher temperatures, there is a higher likelihood of increased cellulose degradation due to prolonged exposure to heat and pressure, resulting in the formation of inhibitory compounds such as furans and organic acids, which can impede enzymatic hydrolysis and fermentation processes. Additionally, excessive thermal treatment might lead to a more pronounced breakdown of hemicellulose, further contributing to reduced sugar yields. Recent research by Brenelli et al. [36] demonstrated a similar trend in steam-exploded biomass, where excessively high temperatures led to reduced sugar recoveries, while Kataria et al. [37] highlighted the significance of optimizing steam explosion conditions to avoid excessive degradation and maximize sugar conversion. The reason behind the adverse effect of enzymatic hydrolysis despite the increased level of xylan solubilization may be attributed of other factors that affect enzymatic hydrolysis, such as substrate crystallinity, hemicellulose and lignin content, S/G ratio, product and enzyme inhibition also as, cellulase loading and reaction conditions. However, the maximum glucan conversion in W4-200 can be attributed to its lower residual lignin content in solid residue, which may have hindered cellulases from inefficiently binding with lignin. In the results, glucan conversion gets reduced by ~ 15 to 21%, while the pretreatment process conducting at a temperature below 190 °C and above the temperature beyond 210 °C respectively. Thus, it has been demonstrated that enzymatic hydrolysis may be restricted beyond a certain point. Additionally, the maximum total sugar concentration was achieved with SA2-200 (70.9%), followed by SA1-190 (65.3%) and W4-200 (50.9%).
The effects of total inhibitor concentration and xylo-oligomers on glucan conversion of unwashed and washed pretreatment residue are shown in Fig. 3. In general, xylo-oligomers were formed by the incomplete hydrolysis of xylan and made up by the xylose backbone connected with arabinofuranosyl, acetyl-groups, ferulic and p-coumaric acid. Gaur et al. [23] and Gao et al. [38] found that the xylo-oligomers with higher DP act as an inhibitors, which detriment the cellulase activity. Though the washing the pretreated residues after steam explosion could remove the soluble oligomers and improved the enzymatic hydrolysis, however, the process is not suitable as the lots of water requirement and loss the sugars during washing. Moreover, xylose concentration in hydrolysate was reduced post washing step by which a very low ethanol titre in the broth after fermentation could be obtained. The result shows that glucan conversion increased from 42.5, 37.1 and 21.6 (unwashed pretreated biomass) to 47.9, 63.7 and 62.4% (washed pretreated biomass) for W4-200, W5-210 and W6-220 respectively. After washing, it was found that the percentage increase in glucan conversion was 4% (W4-200) < 26.6% (W5-210) < 40.8% (W6-220). This pattern is consistent with the formation of xylo-oligomers and other inhibitors, which may affect cellulases activity to variable degrees. However, washing of biomass is ineffective for W1-170, W2-180, W3-180 and W4-190 as the glucan conversion is decreased from 27.4, 26.3, 33.7, 57.0, 65.4 to 12.4, 20.5 and 29.1% respectively. Reduction in the glucan conversion of SA1-190 and SA2-200 post washing suggests that xylo-oligomers coupled with their lowest amount (9.6 and 8.6% of the total hemicelluloses, Table 1) had the least impact on cellulases inhibition. Disappearance of xylo-oligomers in SA1-190 and SA2-200, the inclusion of dilute acid during SE encourages the breakdown of oligomers to monomers that are present in the liquid fraction with digestible cellulose in the solid residue, encouraging enzymatic conversion. Hence, DA assisted SE also co-produced several inhibitory products that impair the subsequent bio-ethanol conversion process, such as the breakdown of glucose into HMF, xylose into furfural, acetic acid, formic acid, phenolics formed from lignin, oligomers, and re-polymerized furans and pseudolignins [32, 39].
The effect of increasing enzyme doses from 1.5 to 5.0 FPU with total sugar concentration in g L–1 was shown in Fig. 4 and it was shown that increasing the enzyme dose can also enhance the sugar concentrations. The reactions were carried out in W4-200 at 15% solid loading using 1.5 FPU to 5.0 FPU of cellulases g–1 biomass. The figure indicates that for 2.0, 2.5, 3.0, 4.0, and 5.0 FPU, respectively, the glucan conversion rose from 42.5 (1.5 FPU) to 45.9, 48.0, 51.0, 55.8, and 58.7%. As increasing the enzyme dosages, the % enlarge in glucan conversion was found to be 3.4% (2.0 FPU) > 5.5% (2.5 FPU) > 8.5% (3.0 FPU) > 13.3% (4.0 FPU) > 16.2% (5.0 FPU).
It could be argued that because xylan or xylo-oligomers were less abundant in pretreatment residue than glucan; further process improvement might result in a greater intensity of glucan conversion. However, it would be preferable to increase sugar concentration while still keeping costs low by utilizing small amounts of the enzyme. It has been reported that increasing the enzyme dose to 1 FPU g–1 WIS causes an increase in ethanol yield. This, in turn, causes an increase in ethanol cost as well as a 5% rise in GHG emissions [20]. In order to reduce the impact of enzymes on the overall conversion, the pretreatment process must be improved. It may be argued that the 1.5 FPU of cellulase g–1 residue would be advantageous for total sugar recovery and ethanol titer and it could be enhanced by adding acid or changing the process conditions. Additionally, careful optimization is necessary for improved economy because there is a trade-off between uses of enzyme vis-a-vis use of dilute acid.
FTIR Analysis
Figure 5 showed the IR spectra of untreated and treated solid residues under various reaction conditions using FTIR. The prominent band at 1200–1000 cm–1 was associated with the structural features of cellulose and hemicellulose. The peak at 1087, 1132, and 1172 cm–1 corresponded to cellulose's C–O–H and C–O–C stretching, and it was exacerbated by an increase in glucan content during steam explosion. Whereas, transition from cellulose’s crystalline to amorphous structure enhanced the band at 894 cm–1 (β-(1→4)-glycosidic linkages of C–O–C stretching). However, at the adsorption peak of 3380 cm–1, O–H stretching of hydrogen bonds was nearly identical for native and pretreated residue. The peak at 2926 cm–1, which is related to C–H stretching within the cellulose's methylene, increased to a certain extent after pretreatment [15].
The adsorption peak at 1720 cm–1was associated with the ester linkage between hemicellulose and lignin as well as the C=O and C–O bonds of the acetyl group in the hemicellulose structure. Considering the results, the steam explosion of rice straw at high pretreatment temperature and by dilute acid addition resulted in decreased xylan content and also found a very less or negligible amount of acetyl group (Table 1). The deacetylation of hemicellulose results in the decrease the intensity of these bands [7, 40]. The band was more pronounce in W1-170, W2-180, and W3-190, while, the intensity of these bands was reduced with increasing severity in W4-200, W5-210, W6-220, SA1-190, and SA2-200. Further, the appearance of adsorption peak at 1720 cm–1 in the SE treated residue also reflects the presence of ester linkages between lignin and hemicellulose. The band at 1624, 1512, and 1438 cm–1 ascribed to aromatic skeletal stretching of lignin, whereas, band at 1657 cm–1and 1723–1715 cm–1 attributed to stretching of C=O conjugated and un-conjugated to an aromatic ring respectively. The intensification of characteristic peak of lignin at 1246 cm–1 and 1327 cm–1 in pretreated biomass might be due to increased lignin concentration, which was also supported by compositional analysis, presented in Table 1. It was observed that the matrix structure might be destroyed and small lignin fragments produced when the ether bonds of the lignin structure were hydrolyzed during pretreatment at high severity. Therefore, due to hydrophobic interactions, the redistribution of lignin may easily congregate into drops within the pretreated residue and by nonproductive binding of cellulase with lignin through hindering the cellulase to attack the surface of cellulose [24].
Mass Balance of the Process and Waste Potential
Figure 6 illustrates the mass balance and distribution of sugars post pretreatment including pretreatment hydrolysate and water-insoluble solid of pretreated slurry and enzymatic hydrolysis at the optimized reaction conditions of water (W4-200) and acid-assisted SE (SA2-200) for the conversion of ethanol (expected yield). About 9.8 kg (~ 98%) of the rice straw (total 10 kg of RS) was recovered after water soaking due to the losses of fine particles and biomass during removal of excess liquid by pressing. The moisture RS was then carried out to a SE reactor with desired temperature and pressure with/or without dilute acid supplementation. For instance, post SE, the total solid recovery for W4-200 and SA2-200 were reduced, i.e., 71 and 61% respectively owing to removal of leftover extractives and xylan solubilization. The pretreated residue of W4-200 comprising 5.2 kg glucan, 1.82 kg xylan, 0.18 kg arabinan and 1.69 kg lignin, whereas, SA2-200 contains 5.45 kg glucan, 0.86 kg xylan, and 2.2 kg lignin. The sugar recovery in W4-200 including cellulose rich solid and xylose rich hydrolysate were 96.5% (for C6 sugar) and 71.9% (for C5 sugars) containing a total of 4.01 kg (C6 sugars) and 1.44 kg (C5 sugars) with respect to native RS. Whereas, the sugar recovery in SA2-200 was 91.7% (for C6 sugar) and 55.9% (for C5 sugars) containing a total of 3.82 kg (C6 sugar) and 1.13 kg (C5 sugars). The losses incurred post pretreatment might be possible to the degradation of sugar, elimination of extractives; ash and incomplete dissolution of disintegrate lignin. The respective pretreated rice straw was further subjected to glucan hydrolysis at 15% WIS loading using 1.5 FPU cellulase g–1 of residue. The maximum glucan conversion, i.e. 42.5% obtained in W4-200 across the water-treated SE and further enhanced to 65.4% in SA2-200 by acid addition during SE. The overall sugar recoveries post pretreatment and enzymatic hydrolysis was 38.5 and 58.7% for W4-200 and SA2-200 respectively. The losses observed during glucan hydrolysis may be attributed to the exclusion of xylo-oligomers formed during the process in the mass closure and also incomplete breakdown of lignin. The sugar monomers (C6 + C5) are co-fermented to produce ethanol via yeast strain. Whereas, the leftover lignin and other holocellulose residue obtained after fermentation and distillation are used in co-generation plant to generate electricity [41]. For instance, 10 kg of RS in W4-200 and SA2-200 yields 1.86 and 2.22 L expected ethanol.
Conclusion
Pilot scale (240 kg day–1 capacity) steam explosion of rice straw conducted at different severity by raising the temperature and by using DA for production of fermentable sugars. The maximum glucan conversion (42.5%) was achieved at 200 °C, which was further intensified to 65.4% by incorporating the 1.1 wt% DA by using 1.5 FPU g–1 RS. Hence, utilization of DA enhances the fermentable sugar and overall glucose recovery and subsequently improved ethanol yields could help in improvement in economical and energy benefits during scale-up process. To quantify the cost benefits, a techno-economic analysis of two scenarios i.e., (i) using enzyme alone or (ii) using acid in pretreatment and enzyme is needed in the future.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Devi, A., Singh, A., Bajar, S., Pant, D., Din, Z.U.: Ethanol from lignocellulosic biomass: an in-depth analysis of pre-treatment methods, fermentation approaches and detoxification processes. J. Environ. Chem. Eng. 9, 105798 (2021). https://doi.org/10.1016/j.jece.2021.105798
Yao, Y., Xu, J.H., Sun, D.Q.: Untangling global levelised cost of electricity based on multi-factor learning curve for renewable energy: Wind, solar, geothermal, hydropower and bioenergy. J. Clean. Prod. 285, 124827 (2021). https://doi.org/10.1016/j.jclepro.2020.124827
Huang, J., Wang, J., Huang, Z., Liu, T., Li, H.: Photothermal technique-enabled ambient production of microalgae biodiesel: mechanism and life cycle assessment. Bioresour. Technol. 369, 128390 (2023). https://doi.org/10.1016/j.biortech.2022.128390
Singh, N., Singhania, R.R., Nigam, P.S., Dong, C.D., Patel, A.K., Puri, M.: Global status of lignocellulosic biorefinery: challenges and perspectives. Bioresour. Technol. 344, 126415 (2022). https://doi.org/10.1016/j.biortech.2021.126415
Goodman, B.A.: Utilization of waste straw and husks from rice production: a review. J. Bioresour. Bioprod. 5, 143–162 (2020). https://doi.org/10.1016/j.jobab.2020.07.001
Tan, J., Yu, D., Yuan, J., Wu, H., Luo, H., Zhang, H., Li, X., Li, H., Yang, S.: Efficient delignification of wheat straw for microbial lipid production enabled by a novel ternary deep eutectic solvent containing ethylene glycol. Fuel 347, 128485 (2023). https://doi.org/10.1016/j.fuel.2023.128485
Semwal, S., Gaur, R., Mukherjee, S., Chopra, A., Gupta, R.P., Kumar, R., Tuli, D.K.: Structural features of dilute acid pretreated Acacia mangium and impact of sodium sulfite supplementation on enzymatic hydrolysis. ACS Sustain. Chem. Eng. 4, 4635–4644 (2016). https://doi.org/10.1021/acssuschemeng.6b00758
Gaur, R., Soam, S., Sharma, S., Gupta, R.P., Bansal, V.R., Kumar, R., Tuli, D.K.: Bench scale dilute acid pretreatment optimization for producing fermentable sugars from cotton stalk and physicochemical characterization. Ind. Crops Prod. 83, 104–112 (2016). https://doi.org/10.1016/j.indcrop.2015.11.056
Lim, J.S., Manan, Z.A., Alwi, S.R., Hashim, H.: A review on utilisation of biomass from rice industry as a source of renewable energy. Renew. Sustain. Energy Rev. 16, 3084–3094 (2012). https://doi.org/10.1016/j.rser.2012.02.051
Sukumaran, R.K., Surender, V.J., Sindhu, R., Binod, P., Janu, K.U., Sajna, K.V., Rajasree, K.P., Pandey, A.: Lignocellulosic ethanol in India: prospects, challenges and feedstock availability. Bioresour. Technol. 101, 4826–4833 (2010). https://doi.org/10.1016/j.biortech.2009.11.049
Khan, M.A., Khan, M.Z., Zaman, K., Naz, L.: Global estimates of energy consumption and greenhouse gas emissions. Renew. Sustain. Energy Rev. 29, 336–344 (2014). https://doi.org/10.1016/j.rser.2013.08.091
Wang, W., Wu, X., Chen, A., Xie, X., Wang, Y., Yin, C.: Mitigating effects of ex situ application of rice straw on CH4 and N2O emissions from paddy-upland coexisting system. Sci Rep. 6, 1–8 (2016). https://doi.org/10.1038/srep37402
Hassan, M.K., Chowdhury, R., Ghosh, S., Manna, D., Pappinen, A., Kuittinen, S.: Energy and environmental impact assessment of Indian rice straw for the production of second-generation bioethanol. Sustain. Energy Technol. Assess. 47, 101546 (2021). https://doi.org/10.1016/j.seta.2021.101546
Kapoor, M., Semwal, S., Satlewal, A., Christopher, J., Gupta, R.P., Kumar, R., Puri, S.K., Ramakumar, S.S.V.: The impact of particle size of cellulosic residue and solid loadings on enzymatic hydrolysis with a mass balance. Fuel 245, 514–520 (2019). https://doi.org/10.1016/j.fuel.2019.02.094
Semwal, S., Raj, T., Kumar, R., Christopher, J., Gupta, R.P., Puri, S.K., Kumar, R., Ramakumar, S.S.V.: Process optimization and mass balance studies of pilot scale steam explosion pretreatment of rice straw for higher sugar release. Biomass Bioenergy 130, 105390 (2019). https://doi.org/10.1016/j.biombioe.2019.105390
Sivagurunathan, P., Raj, T., Mohanta, C.S., Semwal, S., Satlewal, A., Gupta, R.P., Puri, S.K., Ramakumar, S.S.V., Kumar, R.: 2G waste lignin to fuel and high value-added chemicals: Approaches, challenges and future outlook for sustainable development. Chemosphere 268, 129326 (2021). https://doi.org/10.1016/j.chemosphere.2020.129326
Zhao, L., Sun, Z.F., Zhang, C.C., Nan, J., Ren, N.Q., Lee, D.J., Chen, C.: Advances in pretreatment of lignocellulosic biomass for bioenergy production: challenges and perspectives. Bioresour. Technol. 343, 126123 (2022). https://doi.org/10.1016/j.biortech.2021.126123
Kapoor, M., Semwal, S., Gaur, R., Kumar, R., Gupta, R.P., Puri, S.K.: The Pretreatment Technologies for Deconstruction of Lignocellulosic Biomass. In: Singhania, R., Agarwal, R., Kumar, R., Sukumaran, R. (eds.) Waste to Wealth, pp. 395–421. Springer, Singapore (2018)
Sharma, S., Kumar, R., Gaur, R., Agrawal, R., Gupta, R.P., Tuli, D.K., Das, B.: Pilot scale study on steam explosion and mass balance for higher sugar recovery from rice straw. Bioresour. Technol. 175, 350–357 (2015). https://doi.org/10.1016/j.biortech.2014.10.112
Soam, S., Kapoor, M., Kumar, R., Borjesson, P., Gupta, R.P., Tuli, D.K.: Global warming potential and energy analysis of second generation ethanol production from rice straw in India. Appl. Energy 184, 353–364 (2016). https://doi.org/10.1016/j.apenergy.2016.10.034
Zhou, Z., Liu, D., Zhao, X.: Conversion of lignocellulose to biofuels and chemicals via sugar platform: an updated review on chemistry and mechanisms of acid hydrolysis of lignocellulose. Renew. Sust. Energ. Rev. 146, 11169 (2021). https://doi.org/10.1016/j.rser.2021.111169
Yuan, Y., Jiang, B., Chen, H., Wu, W., Wu, S., Jin, Y., Xiao, H.: Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 14, 1–20 (2021). https://doi.org/10.1186/s13068-021-02054-1
Gaur, R., Semwal, S., Raj, T., Lamba, B.Y., Ramu, E., Gupta, R.P., Kumar, R., Puri, S.K.: Intensification of steam explosion and structural intricacies impacting sugar recovery. Bioresour. Technol. 241, 692–700 (2017). https://doi.org/10.1016/j.biortech.2017.05.208
Hsu, T.C., Guo, G.L., Chen, W.H., Hwang, W.S.: Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 101, 4907–4913 (2010). https://doi.org/10.1016/j.biortech.2009.10.009
Chen, H.Z., Liu, Z.H.: Steam explosion and its combinatorial pretreatment refining technology of plant biomass to bio-based products. Biotechnol. J. 10, 866–885 (2015). https://doi.org/10.1002/biot.201400705
Delbecq, F., Wang, Y., Muralidhara, A., El Ouardi, K., Marlair, G., Len, C.: Hydrolysis of hemicellulose and derivatives-a review of recent advances in the production of furfural. Front. Chem. 6, 146 (2018). https://doi.org/10.3389/fchem.2018.00146
Kim, D., Yoo, C.G., Schwarz, J., Dhekney, S., Kozak, R., Laufer, C., Ferrier, D., Mackay, S., Ashcraft, M., Williams, R., Kim, S.: Effect of lignin-blocking agent on enzyme hydrolysis of acid pretreated hemp waste. RSC Adv. 11, 22025–22033 (2021). https://doi.org/10.1039/D1RA03412J
Kucharska, K., Rybarczyk, P., Hołowacz, I., Łukajtis, R., Glinka, M., Kamiński, M.: Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 23, 2937 (2018). https://doi.org/10.3390/molecules23112937
Hu, F., Jung, S., Ragauskas, A.: Pseudo-lignin formation and its impact on enzymatic hydrolysis. Bioresour. Technol. 117, 7–12 (2012). https://doi.org/10.1016/j.biortech.2012.04.037
Shinde, S.D., Meng, X., Kumar, R., Ragauskas, A.J.: Recent advances in understanding the pseudo-lignin formation in a lignocellulosic biorefinery. Green Chem. 20, 2192–2205 (2018). https://doi.org/10.1039/C8GC00353J
Ilanidis, D., Stagge, S., Jönsson, L.J., Martín, C.: Hydrothermal pretreatment of wheat straw: effects of temperature and acidity on byproduct formation and inhibition of enzymatic hydrolysis and ethanolic fermentation. Agronomy 11, 487 (2021). https://doi.org/10.3390/agronomy11030487
Jönsson, L.J., Martín, C.: Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 199, 103–112 (2016). https://doi.org/10.1016/j.biortech.2015.10.009
Kapoor, M., Soam, S., Agrawal, R., Gupta, R.P., Tuli, D.K., Kumar, R.: Pilot scale dilute acid pretreatment of rice straw and fermentable sugar recovery at high solid loadings. Bioresour. Technol. 224, 688–693 (2017). https://doi.org/10.1016/j.biortech.2016.11.032
Hoang, A.T., Nizetic, S., Ong, H.C., Chong, C.T., Atabani, A.E.: Acid-based lignocellulosic biomass biorefinery for bioenergy production: advantages, application constraints, and perspectives. J. Environ. Manage. 296, 113194 (2021). https://doi.org/10.1016/j.jenvman.2021.113194
Sui, W., Liu, X., Sun, H., Li, C., Parvez, A.M., Wang, G.: Improved high-solid loading enzymatic hydrolysis of steam exploded corn stalk using rapid room temperature γ-valerolactone delignification. Ind. Crops Prod. 165, 113389 (2021). https://doi.org/10.1016/j.indcrop.2021.113389
Brenelli, L.B., Bhatia, R., Djajadi, D.T., Thygesen, L.G., Rabelo, S.C., Leak, D.J., Franco, T.T., Gallagher, J.A.: Xylo-oligosaccharides, fermentable sugars, and bioenergy production from sugarcane straw using steam explosion pretreatment at pilot-scale. Bioresour. Technol. 357, 127093 (2022). https://doi.org/10.1016/j.biortech.2022.127093
Kataria, R., Mol, A., Schulten, E., Happel, A., Mussatto, S.I.: Bench scale steam explosion pretreatment of acid impregnated elephant grass biomass and its impacts on biomass composition, structure and hydrolysis. Ind. Crops Prod. 106, 48–58 (2017). https://doi.org/10.1016/j.indcrop.2016.08.050
Gao, X., Kumar, R., Singh, S., Simmons, B.A., Balan, V., Dale, B.E., Wyman, C.E.: Comparison of enzymatic reactivity of corn stover solids prepared by dilute acid, AFEX™, and ionic liquid pretreatments. Biotechnol. Biofuels 7, 1–13 (2014). https://doi.org/10.1186/1754-6834-7-71
Sannigrahi, P., Kim, D.H., Jung, S., Ragauskas, A.: Pseudo-lignin and pretreatment chemistry. Energy Environ. Sci. 4, 1306–1310 (2011). https://doi.org/10.1039/C0EE00378F
Kapoor, M., Raj, T., Vijayaraj, M., Chopra, A., Gupta, R.P., Tuli, D.K., Kumar, R.: Structural features of dilute acid, steam exploded, and alkali pretreated mustard stalk and their impact on enzymatic hydrolysis. Carbohydr. Polym. 124, 265–273 (2015). https://doi.org/10.1016/j.carbpol.2015.02.044
Sharma, A., Kaur, P., Singh, G., Arya, S.K.: Economical concerns of lignin in the energy sector. Clean. Eng. Technol. 4, 100258 (2021). https://doi.org/10.1016/j.clet.2021.100258
Acknowledgements
We are grateful to the IOC-DBT Centre for Advanced Bioenergy Research, Indian Oil Corporation Limited (R&D Centre), Faridabad for providing the necessary facilities and Department of Biotechnology (DBT) India for funding under grant number BT/PB/08/03/2007.
Funding
Open access funding provided by Nord University. This work was supported by Department of Biotechnology (DBT) India under grant number BT/PB/08/03/2007.
Author information
Authors and Affiliations
Contributions
Conceptualization and validation: SS, PS, AS and RK; Methodology: SS, RK and RK; Formal analysis: SS, RK and JC; Writing-original draft preparation: SS and PS; Supervision: RK and AS; Project administration: RK and RG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
All authors have read and agreed to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Semwal, S., Sivagurunathan, P., Satlewal, A. et al. An Efficient and Cost- Effective Pretreatment of Rice Straw Using Steam Explosion: A Pilot Scale Experience. Waste Biomass Valor 15, 1975–1986 (2024). https://doi.org/10.1007/s12649-023-02267-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02267-5