Abstract
This study aims to explore the use of wood ash, a common waste, as an alternative and eco-friendly pulping agent. The first and most important singularity of the study is that wood ash solution, though mild in nature, has proven to be an effective pulping agent (in comparison with the common pulping solution, sodium hydroxide): comparable lignin (45%) and hemicellulose (50%) removal, high crystallinity (72–77%) and high pulp yield (70%) were obtained. Another particularity of this study is that the reported advanced aspects of pulped banana fibres, notably the chemical functionality, crystallinity and crystallite size have been correlated with the chemical composition of the wood ash used: calcium pectinate was formed on the pulped fibre surface. It apparently played a role in enhancing the fibre crystallinity. Moreover, another specialness of this study is that non-conventional factors, which would have significant bearing during industrial scale processing, such as liquor ratio, fibre input size and duration have been investigated—the latter has shown that to attain maximum delignification, small fibre input size at a high liquor ratio of 40 for an extended period of 2 h are necessary. Finally, the pulped banana fibres (which were intended to be used for membrane fabrication for use in water purification system) have depicted their applicability in the fabrication of ultrafiltration membranes by virtue of their aspect ratio, semi-hydrophilicity (due to presence of residual lignin) and projected high mechanical strength (due to high crystallinity and low crystallite size).
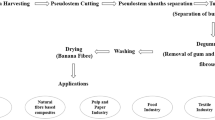
Graphical Abstract
Graphical abstract showing outcomes when pulping with wood ash solution.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Wood ash, a normally discarded by-product, has been valorised as an alternative and green pulping agent, bringing about the concept of Circular Economy. This study showcases one industrially viable option which addresses the research gap – finding a pulping agent giving high pulp yield without compromising the pulp quality: A high pulp yield (70%) coupled with the enhanced cellulose content (85-86%) were obtained. Furthermore, since this proposed pulping method does not require any other chemical additives (as in the most effective and most utilised method: Kraft process), this reduces the chemical cost at larger scales. Thus, this pulping method is more applicable for developing economies. Finally, contrary to the conventional purpose of papermaking, this study proposes an uncommon application (ultrafiltration membrane): this widens the scope of use of the pulped material.
Introduction
Pulping has been mainly used for papermaking. However, other similar applications such as membrane fabrication and film- and mat-making necessitate the use of pulped fibres. In the case of membrane fabrication, numerous studies utilising cellulosic fibres for making membranes have used pulped fibres [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15].
Pulping is basically a process that liberates fibres from a fibrous mass. This is performed by two major mechanisms which occur during this process: delignification (throughout the pulping process) and carbohydrate removal (mainly at the start of the pulping process). Historically, two forms of pulping were performed: mechanical and chemical pulping. Although the yield of pulp is higher for mechanical pulping (85–95%) than chemical pulping (45–50%), the lignin removal efficiency of mechanical pulping (60–70%) was reported to be lower than chemical pulping (90–95%) [16]. Research is still being performed to find better-performing methods to obtain pulp both of good quality (maximum lignin removal) and of highest yield.
Wood ash, a by-product obtained when burning wood, is an alkaline substance having a pH of 9–13 [17]. Since it is obtained from a natural material, wood ash can be considered to be an eco-friendly material. Due to the alkaline nature of wood ash, traditionally, lye (which contains potassium hydroxide) used to be extracted from wood ash. It was utilised mainly as liming agent, fertiliser and neutralising agent of poultry feed, amongst others [17, 18]. Wood ash has also been used as an additive in concrete manufacturing [19], catalyst in biodiesel production [20], secondary raw material in clay bricks [21] and in manufacture of soap [22], amongst others.
Furthermore, to the best of the authors’ knowledge, there is a lack of studies which have reported the use of wood ash as a source of alkali for pulping. The only study using wood ash as a pulping agent has been done by Ekhuemelo et al. [23] who reported that the resulting pulped fibres were of a Runkel ratio close to 1, thereby showing their suitability for papermaking. However, in this research study, other important characterisation features such as the fibre crystallinity (which would give an insight into the fibre strength), physical (fibre length, fibre lumen, fibre diameter and wall thickness) and chemical (lignin, hemicellulose, cellulose contents) characteristics of the pulped fibres, have not been studied.
In order to bridge this gap, the authors propose to investigate into more advanced aspects of pulped fibres such as their degree of fibrillation, their chemical functionality, their cellulose crystallinity and their crystallite size—which are important, since they would provide an insight into the suitability of the pulped fibres for a non-conventional purpose, making membranes. For example:
-
fibres of high degree of fibrillation result in high pore volume and high microporosity of the membranes [24]. When fibres are fibrillated to a high extent, their packing density increases by reason of stronger hydrogen bonds which are formed among the fibrils. These fibrils coalesce together to form denser films or membranes [25]. Consequently, with higher degrees of fibrillation, many minute pores are formed: the membrane formed can thus be used for nanofiltration and ultrafiltration processes (which require very small pore size);
-
the chemical resistance of the membranes under differing water purification conditions can be predicted when fibres of known chemical functionality have been used for the membrane fabrication;
-
fibres of high cellulose crystallinity yield high strength membranes and are inert to chemical and microbial attacks during the performance of the membrane; and
-
fibres of small crystallite size yield higher strength membranes as they contain anti-parallel chains [26] of cellulose II [26, 27].
A peculiarity of the study is that these mentioned advanced characteristics of the pulped fibres (chemical functionality and percentage crystallinity) have been correlated together with the chemical composition of wood ash. Moreover, a section has been included to show the suitability of the pulped fibres for membrane fabrication.
One popular belief is that environment-friendly chemicals, which are usually mild in nature, do not perform as effectively as strong, industrially manufactured chemicals. Hence, one particularity of this study is that the performance of this eco-friendly pulping solution is shown to be equally effective as the common pulping solution. Additionally, the current pulping solution of this study is shown to give a solution to the earlier-mentioned research gap (need for a pulping agent being efficient and giving high yield), resulting into better pulp yields than the common pulping solution.
In addition, to the best of the authors’ knowledge, pulping factors such as concentration of pulping agent, temperature and pressure are usually investigated in studies based on pulping. An ultimate distinctiveness of this study is that non-conventional factors such as liquor ratio, fibre input size and duration have been tested to give important information that will be more closely applicable to industrial scale pulping. Overall, this study gives a holistic analysis of the use of wood ash solution as an alternative and eco-friendly pulping agent, with an aim that the pulped fibres can be used for an atypical purpose, membrane fabrication for use in water purification system.
Materials and Methods
Pulverised banana fibres, extracted from local waste banana pseudo-stems, were wet-grinded using a food wet grinder (Model: FZ001002/F25, Yung Soon Lih Food Machine Ltd., Taiwan) at a fibre consistency of 1% (wt.%).
From a previous work conducted by the authors [28], it was found that the gap width between the two grinding disks in the wet grinding machine was more important than the number of passes through the equipment, for reducing the fibre size. From the investigation, at a gap width of 1600 µm and the number of passes amounting to 40, the mean diameter of the ground banana fibres was found to be 11.91 ± 1.74 µm. During the same investigation, in order to further reduce the fibre size, the gap width needed to be adjusted to 800 µm while the number of passes could be reduced to 30, so as to get ground banana fibres of a mean fibre diameter of 5.11 ± 0.62 µm. For both batches of fibres, their lengths were in the range of 1–5 mm.
For the current study, with a view to determine the effect of fibre input size during chemical pulping reaction, both samples of ground banana fibres (5 µm and 10 µm) were used.
Wood Ash Solution Preparation
The sodium hydroxide (NaOH) concentration during chemical pulping usually falls around 15–20% [29]. To investigate the strength of wood ash solution as a pulping agent and to promote minimal usage of chemicals during pulping reaction, the effect of two smaller concentrations (5% and 10%) other than 15% and 20% were studied.
Wood ash was procured from a local food restaurant from the burning of wood logs. Wood ash solution was prepared by adding a predetermined amount of wood ash (5 g, 10 g, 15 g and 20 g) to 100 ml of distilled water to reach concentrations of 5%, 10%, 15% and 20% respectively.
Chemical Pulping
The wet-ground banana fibres were chemically pulped using the prepared wood ash solution at the different concentrations mentioned above, using a liquor ratio of 10 and 40, a duration of 1 h, 1.5 h and 2 h and a temperature of 120 °C. The reasons for the chosen conditions are: the two chosen liquor ratios are within the ranges used in the studies by Popy et al. [30], Sanjay et al., [31] and Moghaddham et al. [32]. The pulping temperature has been chosen in line with the pulping of banana fibres of the previous study conducted by Cordeiro et al. [33] while the duration of pulping has been extended to 2 h to ensure maximum lignin removal (maximum pulp yield was obtained at 30–45 min by Cordeiro et al. [33]).
Some of the wet-ground banana fibres were also chemically pulped using sodium hydroxide (NaOH) solution–this was to compare the performance of the wood ash solution with respect to the commonly used NaOH solution. This chemical pulping using NaOH solution was done at a NaOH concentration of 20% (using liquor ratio of 40, duration of 2 h and temperature of 120 °C) since pulping with 20% wood ash solution (at a liquor ratio of 40 and duration of 2 h) gave the best results (which are depicted in the Results and Discussion section).
The nomenclature for the fibres analysed are given in Table 1:
Pulp Yield
One important property during pulping reactions is the pulp yield as this will affect the profitability of the process. The pulp yield was calculated using the equation below:
where \({M}_{p}\) is the mass of pulped fibres (in g),
\({M}_{u}\) is the mass of untreated ground fibres (in g).
Analysis of the Pulped Fibres
Scanning Electron Microscopy (SEM)
The surface morphology of the pulped fibres was analysed using a field emission gun scanning electron microscope. The fibre samples were mounted on standard aluminium pin stubs using carbon adhesive tabs. They were sputter-coated using a Quorum Q150R fitted with a gold/palladium target to produce a 5–10 nm conductive coating to facilitate analysis. A JEOL JSM-7800-F field emission scanning electron microscope (FE-SEM) was used for secondary electron imaging, which was operated at an accelerating voltage of 5 kV. Images of the fibre samples were taken at five magnifications (× 500, × 1000, × 5000, × 10,000, × 30,000) at five different locations of the sample holder.
The fibre diameter was measured using the image processing software, ImageJ. At least 25 images for each fibre sample were analysed. 3-sigma method was then used to determine outliers in order to find the mean and standard deviation excluding outliers.
Fourier Transform Infrared Spectroscopy (FTIR)
To get an insight into the chemical functionality of the pulped fibres, FTIR analysis using a FTIR Spectrometer (model: Bruker Optics) in the range of wavenumbers from 400 to 4000 cm−1 at a resolution of 4 cm−1 and with a sample scan time of 24 scans, was conducted. Before performing any interpretation, the FTIR graphs obtained were normalised.
X-ray Diffraction (XRD)
The fibre cellulose crystallinity which is linked to the mechanical strength of fibres can be determined by X-Ray Diffraction (XRD) [34]. The pulped fibres, in the form of powder, were analysed using an X-Ray diffractometer (model: Bruker D2 Phaser) fitted with a 1-dimensional LynxEye detector. A copper X-ray source (Kα = 1.54184 Å) was used at a voltage of 30 kV and a current of 10 mA, with Kβ radiation suppressed by means of a 0.5 mm thick nickel filter. XRD patterns were recorded over a range of 5°–60° (2θ) with a step size of 0.02°, with a maximum intensity of 3000 counts and an equivalent step time of 57.6 s per step. Sample rotation was set at 15 rpm.
OriginPro software (version 8.0) was used to remove the background noise (having an intensity of around 300 counts) by performing a baseline correction at 300 counts.
Percentage Crystallinity
The percentage crystallinity was then computed using the Segal crystallinity method, as per Eq. (2) (Segal et al., 1959):
where: \(CrI\) stands for percentage crystallinity (%), \({I}_{200}\) is the intensity at the highest XRD peak [at (200)], \({I}_{min}\) is the minimum intensity occurring before the highest XRD peak [between the (110) and (200) peak, at around the 2θ value of 18°].
Crystallite Size
Crystallite size was calculated using the Scherrer equation (Scherrer, 1918), as shown in Eq. (3):
where:
\(D\) is the crystallite size (Å),
\(K\) is a constant depending on the crystal shape (taken to be 0.94 for this study),\(\lambda\) is the incident beam wavelength (1.54 Å),\(\beta\) is the full width at half maximum (FWHM) of XRD peaks (in radians),\(\theta\) is the position of the XRD peak (half the value of the plotted value) (in radians).
Interplanar Spacing
The interplanar spacing (also termed as the ‘d-spacing’) was calculated, applying Bragg’s law, using Eq. (4) [35]:
where: \(d\) is the interplanar spacing (Å), \(n\) is an integer (taken to be 1 for this study), \(\lambda\) is the incident beam wavelength (1.54 Å), \(\theta\) is the position of the XRD peak (half the value of the plotted value) (in radians).
Fibre Characterisation: Component Analysis
With a view to support the data obtained from SEM, FTIR and XRD analyses, the following component analysis was done:
-
(1)
the Kappa number, which is a common quantitative measure used in pulping reactions to measure the extent of lignin removed, was determined according to the ISO standard ISO 302:2015;
-
(2)
from the residue in step (1), the hemicellulose content was determined using the method in the work of Park et al. [36]. It was calculated from the difference of the mass of fibres in step (1) and the mass following the hemicellulose determination treatment;
-
(3)
using the residue in step (2), the ⍺-cellulose content was determined as per the TAPPI standard T 429 cm-10; and
-
(4)
using the residue in step (iii), the ash content was determined using the TAPPI standard T 211 om-02.
Results and Discussion
Characterisation of Wood Ash
Energy dispersive spectroscopy (EDS) has been used to get an insight into the elemental composition of the alternative chemical to pulping used in this study, wood ash. Table 2 gives the weight percent of the elements detected by the use of this technique.
From Table 2, the elements oxygen (O) and carbon (C) are in majority showing that there are still combustible materials in the wood ash. The next element in abundance is calcium (Ca) and then potassium (K). Thus, when wood ash was added to water, calcium hydroxide and potassium hydroxide have been formed in majority. In much lesser amounts, sodium hydroxide must have been formed as well.
Both calcium hydroxide and potassium hydroxide are considered as strong bases/alkalis by reason of their high dissociation constants (Kb): Calcium hydroxide = 0.0037; potassium hydroxide = 0.3 [37, 38]. Therefore, the alkaline treatment of the fibres in this study is due to these two strong bases.
Effect of Concentration of Wood Ash solution on Fibres
Chemical pulping is done on fibres with an aim to remove undesired amorphous constituents such as lignin, wax, pectin and hemicellulose. During this study, chemical pulping of ground untreated banana fibres has been performed using an alternative pulping agent, namely, wood ash solution. The strength of a particular reagent can be determined by investigating the effect of its concentration on the effective removal of the amorphous constituents of the natural fibres. Moreover, from an engineering point of view, the concentration of a reagent plays an important role since this will affect the scaling up operations. This section covers the effects of concentration of wood ash solution on the surface morphology (through SEM images), chemical functionality (through FTIR graphs) and crystallinity (through XRD diffractogram) of the ground banana fibres.
SEM Analysis: Effect of Wood Ash concentration on Surface Morphology
Figure 1 shows a selection of the SEM images of the treated fibres at varying wood ash solution concentrations. From the figure, particles resembling small nodules attached to the fibres surface can be noticed (indicated by white circles). Visually, the number of these nodules can be seen to generally increase as the concentration of wood ash solution increases from 5 to 10%. Then, it decreases slightly as the concentration of wood ash solution increases from 10 to 20%.
From the EDS results (Sect. 3.1), the presence of calcium in the wood ash used was shown. Upon dissolution of the wood ash in water to form the wood ash solution, it can thus be inferred that calcium hydroxide was formed. Therefore, during the pulping reaction, the calcium ions in the wood ash solution have reacted with the pectin present on the fibre surface, yielding calcium pectinate. This is confirmed with the SEM results whereby nodules can be observed, which are most certainly calcium pectinate.
This is because when calcium ions come in contact with pectin molecules on the fibre surface, they react with the carboxylate groups of the polygalacturonic acid groups of the latter, thereby forming calcium pectinate, appearing as nodule-like particles [39]. As per the work of Sedan et al. [40], these nodule-like particles occur especially when the calcium ions form bonds with oxygen atoms on adjacent pectin chains: this forms a fixation and thus form a stable egg-box structure which attaches itself to the fibre surface [40]. Thus, these nodules can be observed during SEM analysis.
Ramasawmy et al. [27], Rahman and Azahari [41], Silva et al. [42] and Ciobanu et al. [43] also reported the presence of similar particles when fibres were immersed in calcium hydroxide solution and these particles were attributed to calcium-rich nodules. Moreover, Troedec et al. [44] further reported that this deposition of calcium-containing nodules takes place when the fibres are subjected to calcium ions in an alkaline medium. Therefore, this supports the fact that apart from the calcium hydroxide, potassium hydroxide was also present in the wood ash solution, thereby resulting in a high pH [which ranged from 12.07 (for 5% wood ash solution) to 13.29 (for 20% wood ash solution) for the present study].
Degree of Fibrillation
Furthermore, it is worthwhile to point out that the highest degree of fibrillation is attained when using the highest wood ash solution concentration (Fig. 1d); this is almost similar to the degree of fibrillation noted when using the control sodium hydroxide concentration: flattened structures (indicated by white arrows), due to internal fibrillation of fibres, can be noted in both FN 4 and FN 5 in Fig. 1d, e.
FTIR Analysis: Effect of Wood Ash Concentration on Chemical Functionality
Figure 2 shows the FTIR graphs of untreated and treated banana fibres using varying concentrations of wood ash solution. From the full FTIR spectra (available in supplementary file), the main peaks that were identified, have been given in Table 3, along with their respective bond assignments.
a FTIR graphs for untreated and treated banana fibres at 5%, 10%, 15%, 20% wood ash solutions and 20% NaOH solution (as control); b Variation of FTIR absorbance heights for lignin for FN 1 to FN 4; c Variation of FTIR absorbance heights for pectin for FN 1 to FN 4; d Variation of FTIR absorbance heights for cellulose for FN 1 to FN 4 (cellulose I wavenumbers are represented by black lines and filled circle markers while cellulose II wavenumbers are represented by grey lines and open circle markers)
Analyses for the different non-cellulosic components including lignin, hemicellulose and pectin as well as for the cellulose structures (cellulose I and cellulose II) are outlined below.
Lignin
From the FTIR spectra and from Table 3, the presence of lignin has been noted from the peaks at the wavenumbers 1244, 1510 and 1598 cm−1. Overall, a general decreasing trend in the peak heights (attributed to the wavenumbers 1244, 1510 and 1598 cm−1) with an increase in wood ash concentration was observed.
A decrease of 45% was noted for the lignin peaks when comparing the absorbance heights of untreated fibres and the 20% wood ash treated fibres. The peaks for 20% wood ash solution-treated fibres are less protruding (absorbance height at 1244 cm−1: 0.0062; at 1510 cm−1: 0.0089; at 1598 cm−1: 0.0099) as compared to those for the untreated fibres (absorbance height at 1244 cm−1: 0.0113; at 1510 cm−1: 0.0110; at 1598 cm−1: 0.0180).
As for the lower wood ash concentrations (5%, 10% and 15%), a decrease in the absorbance heights of the lignin peaks of 13% to 40% can be noted. Therefore, it can be inferred that at a wood ash solution concentration of 20%, the extent of lignin removal is highest. This corroborates with the Kappa number (in Table 4 which gives the chemical composition of the fibres analysed) whereby it is lowest for FN 4.
Moreover, the absorbance heights for the 20% wood ash solution-treated fibres are comparable to those of the control NaOH-treated fibres (absorbance height at 1244 cm−1: 0.0108; at 1510 cm−1: 0.0091; at 1598 cm−1: 0.0032). Thus, it can be inferred that the effect of wood ash solution on the banana fibres is comparable to that of the NaOH solution. This is supported by the similar Kappa numbers (Table 4) obtained for FN 4 and FN 5.
Analysis of Kappa Number
Kappa number is an important quantitative measure for the pulping process. It provides an insight into the extent of lignin that has been removed during the pulping. Alaejos et al. [48] who studied different kinds of pulping methods on holm oak trimmings reported comparable Kappa numbers with those of the present study (30.7 for highest wood ash concentration): from soda-anthraquinone pulping, the resulting Kappa number was 34.1–49.4 while from Kraft pulping, the Kappa number obtained was 27.3–33.2.
The highest lignin removal (45%) has been noted at the highest wood ash solution concentration (20%). From the mechanism of delignification, it can be inferred that at this concentration, both initial and bulk phases of delignification have occurred. This is because during the initial phase of delignification, around 15–20% lignin is removed due to the cleaving of α and β aryl ether bonds in phenolic units of lignin. During the bulk phase, a larger extent of lignin (50–60%) is removed due to the cleaving of β aryl ether bonds in non-phenolic units of lignin (Gierer, 1980).
Hemicellulose
Hemicellulose can be detected at the wavenumber 1730 cm−1. From the FTIR spectra (Fig. 2a), the peaks at 1730 cm−1 are almost imperceptible for the fibres treated with wood ash solution as well as with NaOH solution. Thus, at all the concentrations of wood ash solutions, the majority of hemicellulose has been removed.
This is because of the following two mechanisms of carbohydrate removal (in this case, the carbohydrate is hemicellulose) during pulping reactions [49]:
-
(1)deacetylation occurs spontaneously when alkali is added to the fibres. Thus, a major part of carbohydrates turns into acetic acid at the start of the pulping reaction, which then dissolves into the pulping liquor; and
-
(2)the carbohydrates are further removed when peeling, and stopping reactions occur when the temperature of around 100 °C is reached during the pulping reaction.
This corroborates with the data in Table 4 which shows that the hemicellulose contents for the fibre samples FN 1 to FN 5 have decreased from 10.8% (untreated fibres) to 3.5–10.0% (for the treated samples FN 1 to FN 5). Thus, some hemicellulose has still remained in the treated samples.
Likewise, Paixao et al. [50] who studied the sugarcane bagasse delignification with potassium hydroxide, reported that hemicellulose was reduced from 28.96 to 19% when using a concentration of 10%. Moreover, Ramakrishna and Sundarajan [51] reported that the maximum hemicellulose removal was 69% with the most aggressive chemical treatment (NaOH treatment). Thus, this confirms that chemical treatments do not completely remove hemicellulose in fibres.
Pectin
Pectin can be observed through the peaks at the wavenumbers 1234, 1445 and 1648 cm−1. From the FTIR spectra, no peaks can be discerned at 1648 cm−1. At the wavenumbers 1234 and 1445 cm−1, with an increase in the wood ash concentration, the peaks are less and less predominant. This is supported by the decline in the absorbance heights: at 1234 cm−1, the absorbance heights decrease from 0.0095 to 0.0091 to 0.0092 to 0.0056 as concentration of wood ash solution increases from 5 to 10% to 15% to 20%. For the same increase in concentration of wood ash solution, at 1445 cm−1, the absorbance heights decrease from 0.022 to 0.017 to 0.031 to 0.017. Therefore, pectin gets gradually removed as concentration of wood ash solution rises.
However, a relatively higher content of pectin can be noted from the fibres treated with highest wood ash concentration in comparison with those treated with the control NaOH solution (absorbance height at 1234 cm−1: 0.0039; at 1445 cm−1: 0.0082). This is because of the calcium ions in predominance in the wood ash solution: as mentioned earlier in Sect. 3.2.1, calcium ions react with the pectin to form calcium pectinate which remains attached to the fibre surface. Thus, this corroborates with the SEM observations.
Cellulose I and Cellulose II Structures
Cellulose I structure gets transformed to cellulose II structure in the presence of a highly alkaline solution (pH of 13–14). From the FTIR spectra, the presence of cellulose I can be detected from the peaks at the wavenumbers 897, 1111 (strong peak), 3270 and 3350 cm−1 while cellulose II can be detected from the peaks at 1111 (shoulder peak), 1315, 1335 and 1375 cm−1 [45, 52].
At 1111 cm−1, strong peaks can be noticed, thereby showing the presence of cellulose I structure. The absorbance heights, giving the relative extent of the component being analysed, are observed to increase from FN 1 (0.069) to FN 3 (0.085), with a notable decrease to 0.048 for FN 4. Moreover, it is worthy to state that a shoulder peak at this wavenumber was noted for the NaOH-treated fibres, thereby indicating the presence of cellulose II structure.
The same trend is also observed for the peaks at the wavenumbers 897, 3270 and 3350 cm−1: at 897 cm−1, the absorbance height shows a comparative increase from 0.016 (FN 1) to 0.017 (FN 3) and then a relative decrease to 0.0089 (FN 4). Likewise, at 3270 cm−1, the absorbance height increases from 0.050 (FN 1) to 0.057 (FN 3) and then decreases to 0.036 (FN 4). Similarly, at 3350 cm−1, the absorbance height increases from 0.053 (FN 1) to 0.059 (FN 3) and then decreases to 0.037 (FN 4). It can be thus inferred that some cellulose I structure has been degraded at the 20% wood ash concentration. However, from Table 4, the cellulose content shows a general increase from FN 1 (79.5%) to FN 4 (85.8%): this could be because it accounts for both cellulose I and II structures.
Jimenez et al. [53] reported that the resulting cellulose content was 73.74% following Kraft pulping and 70.00% following soda pulping. Lim et al. [54] also reported that the cellulose content after performing ionic liquid pulping was 73.8%. Additionally, Paixao et al. [50] further reported that the cellulose content rose from 42.43 to 79.8% during potassium hydroxide pulping. Therefore, this shows that wood ash pulping resulted in comparable, if not, higher cellulose contents of the banana fibres in this study.
From the FTIR spectra, peaks can be perceived at the wavenumbers 1315, 1335 and 1375 cm−1, thereby showing the presence of cellulose II structure in the chemically treated fibres. Similar to the trend for cellulose I structure, the absorbance heights at these wavenumbers increase for FN 1 to FN 3 and then decrease for FN 4 and 5. However, contrary to the fibres treated with wood ash solution, a peak at 893 cm−1 can be noted for the NaOH-treated fibres—therefore, there could be a greater extent of cellulose II structure in the latter as compared to the wood ash-treated fibres.
Overall, there could be a mixture of cellulose I and II structures in all the chemically-treated fibres, though cellulose II structure being predominant in the NaOH-treated fibres. In the next section, the presence of cellulose II will be confirmed in the XRD analysis.
XRD Analysis: Effect of Wood ash Concentration on Fibre crystallinity
Figure 3 shows the XRD diffractograms for the pulped fibres and the variation of percent crystallinity and crystallite size. From the XRD diffractograms, the major peaks obtained are at 2θ values of 15.5°–15.8° and 22.3°–22.5° for FN 1 to FN 4, indicating the presence of cellulose Iβ structure (since the fibres originate from a plant material). As per French [55], the peaks around 15.5° have Miller indices (1–10) and (110), while the peaks around 22.5° have Miller index (200).
a–e XRD diffractograms for banana fibres [FN1, FN 2, FN 3, FN 4] pulped using wood ash solution at different concentrations and using NaOH as control [FN 5]; f Variation of percentage crystallinity and average crystallite size for banana fibres treated at different concentrations of wood ash solution and using NaOH as control
Other shorter peaks can be observed for FN 1 to FN 5 at the 2θ values of 25.5°, 35°, 37.5°, 43.3°, 52.5° and 57.5°: these are attributed to the Corundum particles from the wet grinding disks, used to grind the banana fibres in this study, prior to the pulping reaction. Chen et al. [56] reported the peaks at the same 2θ values for the Corundum material.
It is noteworthy to state that there is an additional peak at the 2θ values 29.4°–29.7° for FN 1 to FN 5, supporting the earlier observations of calcium pectinate on the surface of the fibres. As reported by Augustine et al. [57], XRD analysis of calcium pectinate reveals peaks at the same 2θ value of 29.4°–29.7°.
Moreover, for FN 5, from the XRD diffractogram, the cellulose peaks are shifted to the 2θ values of 11.8° and 20.1°, implying the presence of cellulose II structure [55] in FN 5 rather than cellulose I structure. This supports the FTIR results which also showed that cellulose II was present in the NaOH pulped fibres.
Concomitantly with the previous Sect. 3.2.2, a general increase in the percentage crystallinity can be noted, with the two highest percentage crystallinity noted for the 20% wood ash (72.2%) and for the 20% NaOH control solution (75.8%). This is expected as the lignin and hemicellulose get removed, the cellulose gets more and more exposed. Thus, this results in increasing the percentage crystallinity. However, such increase in percentage crystallinity is not usually expected for fibres treated in a calcium hydroxide medium. This is because a slight decrease in the percentage crystallinity has been noted in such conditions by a few studies [40, 44]. Therefore, it can be inferred that the calcium ions in the wood ash solution did not degrade the cellulose structure of the fibres in the present study.
From Fig. 3, it can be seen that as concentration of wood ash increases (FN 1 to FN 4), the crystallite size remains quasi-constant (22.2–24.1 Å). A slightly lower crystallite size is noted for the control NaOH solution (19.7 Å). Therefore, NaOH solution caused some refinement in the crystallite size as compared to wood ash solution. Correlating with the FTIR analysis, this could explain the more conspicuous transformation of cellulose I to II structure (peak of 897 cm−1 shifting to 893 cm−1) for NaOH-pulped fibres as compared to the wood ash pulped fibres.
When crystals become smaller, their packing density increases and thus, the interplanar spacing (d-spacing) is expected to decrease. From the data calculated for XRD results (data in supplementary material), the d-spacing of the fibres from the control NaOH solution (7.38 Å) is greater as compared to the fibres treated with wood ash solutions (ranging from 5.70 to 5.79 Å). Therefore, the d-spacing of the NaOH-treated fibres has not decreased as expected. This contradiction can be explained by the aggregation of crystals, causing the d-spacing to appear larger than expected.
Factors to be Considered for Scale up Processes
The aim of performing a pulping reaction at lab scale is to get a basis to be able to bring the process workable at the industrial scale. Other factors that are important in large scale processes are liquor ratio, fibre input size and duration. Liquor ratio is a parameter of good importance since it entails the amount of chemicals to be procured for the process. Thus, the higher the liquor ratio, the more chemical(s) is/are needed for the chemical process.
As for the fibre input size, at industrial scale, the input size of fibres in a pulping process is not always homogeneous. This can affect the chemical process. Moreover, the smaller the fibres, the more surface area is exposed, the better the chemical penetrates the fibre interlayers, the more efficiently the undesired components are dissolved away.
In order to investigate the effects of these different factors, the surface morphology (through SEM analysis), chemical functionality (through FTIR analysis) and fibre crystallinity (through XRD analysis) have been analysed.
Analysis of Surface Morphology
Figure 4 shows the SEM images of the pulped fibres at two different conditions of liquor ratios and two different conditions of fibre input size.
Comparing Fig. 4a, b, fibre surfaces in both SEM images appear clean with only some random particles on the surface. However, from Fig. 4a, single fibres can still be noted (indicated by the white circles) while in Fig. 4b, individual fibres cannot be easily discerned (the few fibres that can be discerned are indicated by white circles). Thus, for Fig. 4b, the chemical wood ash solution treatment of higher liquor ratio has fibrillated the fibres internally and externally. Such type of loosened structure has also been reported by Megra et al. [58] who performed kraft pulping on Melia azedarach wood.
Comparing Fig. 4c, d, more particles (indicated by white rectangles) can be discerned on the fibre surface of Fig. 4c as compared to those at d. Thus, it is confirmed that the wood ash solution has cleaned the surface better for the smaller input size as compared to the larger fibres. Therefore, the fibre input size is certainly an important factor to take into consideration during pulping.
Analysis of Chemical Functionality
Figure 5 gives the FTIR graphs for two different liquor ratios (Fig. 5a, b) and for two different fibre input sizes (Fig. 5b, c).
In the case of large batch scale processes, the duration of a chemical treatment is important to ensure the good quality of products. In order to analyse the effect of duration of the process, Fig. 5a, b further gives the different FTIR spectra over time.
Overall, upon using liquor ratio of 40, better removal of non-cellulosic fibre constituents has been noted; thus, a liquor ratio of 40 has been used for comparison of the two different fibre input sizes.
Duration of Pulping
From Fig. 5a, the peaks for lignin (at 1235 cm−1, 1510 cm−1 and 1598 cm−1) become gradually less prominent as duration of pulping increases. Therefore, lignin is gradually removed as pulping progresses. Therefore, the pulping process should be around 2 h so that maximum lignin can be removed from the fibre surface. This is similar to the study conducted by Popy et al. [30] who performed pulping by using potassium hydroxide: the study reported that the minimum Kappa number was obtained at the maximum time, 2 h.
As for hemicellulose, at 1730 cm−1, the peak for the untreated fibres is very prominent while the peaks for the treated fibres are all barely noticeable in Fig. 5a. This depicts that hemicellulose is removed at the start of the pulping process.
As for pectin, depicted by wavenumbers 1234, 1445 and 1648 cm−1, no peaks can be discerned at 1234 and 1648 cm−1 in Fig. 5a. However, a peak can be noticed at 1445 cm−1 at the two-hour treatment, thereby indicating that a small amount of pectin is still present at the end of the pulping treatment. This corroborates with the findings obtained in the earlier Sect. 3.2.2, whereby it was explained that calcium ions from the wood ash solution react with pectin to form calcium pectinate which remains attached to the fibre surface.
As for cellulose, no peaks are present at 893 cm−1 but some can be noted at the wavenumbers 1315, 1335 and 1375 cm−1. This depicts the presence of some cellulose II structure in the chemically-treated fibres over time. As for cellulose I structure, taking 897 cm−1 as reference, from Fig. 5a, the absorbance height increases show a continuous decrease from 0.012 to 0.0089—in the latter case, this shows that cellulose was getting slightly damaged during the reaction. Adding to this, in the next section, it will be shown from the XRD results that there has been no shift in the XRD peaks to confirm the presence of cellulose II. Thus, this again confirms that cellulose I was slightly damaged and not transformed to cellulose II structure.
Liquor Ratio
Comparing Fig. 5b, in terms of lignin, peaks of higher intensity at the wavenumbers 1510 (absorbance height: 0.0039) and 1598 cm−1 (FTIR absorbance height: 0.014) can be noted for FN 6 as compared to FN 4 (peak at wavenumber 1510 cm−1 is not discernible and absorbance height at 1598 cm−1 is 0.0099). This shows that there is higher extent of lignin in the fibres treated at lower liquor ratio. This agrees with the Kappa number for FN 6 and FN 4 (in Table 4).
As for hemicellulose, no peaks are noticeable for both liquor ratios, thereby indicating that hemicellulose was removed in both treatments of different liquor ratios. However, from Table 4, some hemicellulose is found present in FN 6 (9.8%) and in FN 4 (5.0%). Nevertheless, more hemicellulose is removed at the higher liquor ratio.
As for pectin, although no peaks were observed at the wavenumbers 1234 and 1648 cm−1, a peak having absorbance height of 0.019 can be noticed for FN 6 while the absorbance height at the same wavenumber is less for FN 4 (0.017). Hence, more pectin has been removed at the higher liquor ratio treatment.
In the case of cellulose, the extent of cellulose I decreases when liquor ratio is increased from 10 to 40: the absorbance height for FN 6 is higher than that for FN 4 (at 1111 cm−1, FN 6: 0.078, FN 4: 0.048; at 897 cm−1, FN 6: 0.022, FN 4: 0.0089; at 3270 cm−1, FN 6: 0.058, FN 4: 0.036; at 3350 cm−1, FN 6: 0.058, FN 4: 0.037). Similarly, for cellulose II structure, the absorbance heights at 1315 and 1335 cm−1 are lower for FN 4 (0.020–0.024) than for FN 6 (0.025–0.029). Thus, cellulose gets degraded at higher liquor ratio. Yet, this disagrees with the cellulose contents obtained for FN 6 (79.0%) and for FN 4 (85.8%) which shows that at higher liquor ratio, the cellulose content is higher.
Larger Fibre Input Size
From Fig. 5c, in terms of lignin, at the wavenumber 1245 cm−1, the FTIR absorbance heights (0.026) for the larger fibre input size are greater than that for the smaller fibre input size (at 1245 cm−1, absorbance height for larger fibres: 0.026, absorbance height for smaller fibres: 0.0088; at 1598 cm−1, absorbance height for larger fibres: 0.020, absorbance height for smaller fibres: 0.0099). This shows that a greater extent of lignin is removed in the smaller fibres which could be explained by the larger surface area exposed.
Correlating with Kappa Number
This is supported by the Kappa number (in Table 4) for FN 7 (39.0) and FN 4 (30.7). Through this quantitative indicator used in pulping processes, it can be noticed that more lignin has been removed in FN 4 as compared to FN 7.
As for hemicellulose, from Fig. 5c, no peaks at 1730 cm−1 are noticeable. Thus, hemicellulose gets removed almost completely during the pulping reaction for both fibre sizes. From Table 4, it can be observed that a slightly higher extent of hemicellulose is found in FN 7 (7.3%) than in FN 4 (5.0%).
However, for pectin, from Fig. 5c, despite there are no discernible peaks at 1648 cm−1 for both fibre sizes, some peaks of low absorbance heights (0.006–0.017) can be observed for the smaller fibres at 1234 and 1453 cm−1. Thus, it can be inferred that calcium pectinate has remained attached to the surface of the smaller fibres.
Finally, as for both cellulose I and II, from Fig. 5c, the absorbance heights at the wavenumbers 897, 1111, 3270, 3350, 1315, 1335 and 1375 cm−1 are greater in general for the larger fibres as compared to the smaller ones. This observation corroborates with the percent crystallinity given in the next Sect. 3.3.3 (% crystallinity of larger fibres is 77.8% and for smaller fibres it is 72.2%). Thus, this confirms that there is a higher extent of cellulose in the larger fibres than in the smaller ones. However, this is not much in line with the cellulose content in Table 4 as the cellulose content of FN 7 (80.3%) is slightly less than that of FN 4 (85.8%).
Analysis of Fibre Crystallinity
Figure 6 shows the percentage crystallinity and average crystallite size for the fibres treated under different conditions and gives XRD diffractograms for FN 6 to FN 8. From the XRD diffractograms, similarly as in the earlier Sect. 3.2.3, for FN 6 and FN 7, there are two major cellulose Iβ peaks at 2θ values of 15.7° [an overlap of two peaks having Miller indices (1–10) and (110)] and 22.3° [having Miller index (200)]. For FN 8, these two peaks are shifted to the 2θ values of 8.8° and 19.5°, implying the presence of cellulose II in this fibre sample. The rest of the peaks identified (at 2θ = 25.5°, 35°, 37.5°, 43.3°, 52.5° and 57.5°) result from contamination of the particles from the wet grinding disks. Moreover, as in the earlier Sect. 3.2.3, an additional peak at 28.9°–29.7°, representing calcium pectinate, can be noted.
Percentage Crystallinity
The highest percentage crystallinity values have been obtained for the larger fibre input size for both wood ash solution and NaOH solution (78% and 82% respectively) as well as the higher liquor ratio treatment. Therefore, it can be inferred that the cellulose could have been slightly degraded for the smaller input sizes, thereby decreasing the percentage crystallinity. This agrees with the FTIR absorbance heights obtained, as smaller peaks for cellulose for the smaller fibre input size (that is, with lower absorbance height values) were obtained.
Li et al. [59] reported that glucose was formed as a result of cellulose degradation. The XRD analysis of glucose gives a major peak around 20° [60]. From Fig. 6a–c, since the peaks near 20° are broad, it could be indicating a mixture of cellulose and glucose, thereby confirming that cellulose has indeed been degraded during the pulping reaction for the smaller fibre input size (FN 4). Thus, this further supports the hypothesis of cellulose being degraded during the reaction.
Additionally, to again support that the cellulose I was slightly degraded during the reaction and not transformed to cellulose II structure, from Fig. 6a, b, no shift in the XRD peaks to the 2θ values of 19° (which would have indicated the presence of cellulose II as per the work of French [61]) could be noted. Hence, no cellulose II was present and thus, cellulose I was not transformed to cellulose II during the pulping of the smaller sized-fibres (FN 4).
Crystallite Size
As for the crystallite size, it can be noticed from Fig. 6 that the crystallite sizes for fibres from the wood ash solution treatment (21–25 Å) are generally smaller than those from the NaOH treatment (19–42 Å). This means that the crystallites have been more refined following the wood ash solution treatment. This narrow crystallite size distribution obtained for the wood ash pulping process provides evidence that alkalization has taken place [62]—(to note that alkalization brings about a ‘peeling off’ of cellulose chains accompanied by their rearrangement: this, in turn, causes large crystallites to disintegrate into smaller ones).
This peeling reaction, which occurs as part of the carbohydrate removal mechanism (referred to in the earlier Sect. 3.2.2) [49], has occurred due to the OH− ions present in the wood ash solution: the OH− ions react with the reducing ends of carbohydrate molecules such as glucomannan (present in hemicellulose) causing a breakage in the molecular chain [59]. Thus, crystallites formerly consisting of long carbohydrate molecular chains, now comprise of smaller molecular chains: hence, this explains why crystallites become smaller following pulping reaction.
Moreover, the fact that the crystallites of the wood ash-treated fibres are generally smaller (FN 1–FN 4: 22.2–24.1 Å; FN 6: 21.9 Å) than those of the NaOH-treated fibres (FN 8: 42.2 Å) is because there has been possible aggregation of crystallites for FN 8 during the XRD analysis.
Pulp Yield
Pulp yield is an important factor in large scale pulping processes since this often determines the extent of profit to be obtained. Table 5 below summarises the pulp yields obtained for fibres pulped under all different conditions applied in this study.
From Table 5, overall, low pulp yields have been obtained for NaOH pulping as compared to wood ash solution pulping—the pulp yields obtained for the latter are higher than expected since normally during chemical pulping (especially when using NaOH as one of the pulping agents), the pulp yield falls around 50% [30, 48, 53, 58, 63].
Comparing the pulp yields among the various concentrations of wood ash solutions used, a slight decrease can be noted in the pulp yield as concentration of wood ash solution is increased from 5 to 20%. However, the pulp yield is still high (70%) at the highest concentration of wood ash solution. Moreover, it is noteworthy to state that a low pulp yield was obtained for FN 7 since these larger fibres, when untreated, had a large lignin content of 21.7% (the smaller fibres, when untreated, had a smaller lignin content of 11.3%): this caused a higher mass loss during pulping, hence leading to a low pulp yield.
In conclusion, using wood ash solution as a pulping agent results in an appreciably high pulp yield. Thus, this makes wood ash solution an attractive option as a pulping agent.
Discussion of Findings
The aim to pulp the banana fibres in this study is to use them principally as a raw material for membrane fabrication to be used, in turn, in water purification applications. The following points have been gathered to show the suitability of the pulped fibres to such an application:
-
from the SEM images, fibres of diameter of 5.11 ± 0.62 µm and of length of around 50–100 µm were obtained: using the data from Sharma et al. [64], the projected pore size of the membrane is around 15 µm while the aspect ratio of the fibres (10–20) ensures high degree of polymerisation among the fibres, thereby assuring high inter-fibre bonding;
-
from the FTIR results, hemicellulose has been widely removed while 45% lignin has been removed (for the optimum wood ash pulping treatment): this shows the presence of residual lignin, which is reported to confer high wet mechanical strength to membranes [65]. Also, the hydrophobic lignin and the hydrophilic cellulose will make the membrane semi-hydrophilic, which is a desirable property in water purification applications; and
-
from the XRD results, the high percentage crystallinity obtained (around 72% for the optimum wood ash pulping treatment) confirms that the membrane made out of these pulped fibres will be of high mechanical strength.
Therefore, the pulped fibres can be used without any post treatment to fabricate an ultrafiltration membrane or as support membrane layer in a reverse osmosis setup.
Conclusion
Wood ash solution, an eco-friendly reagent, has been successfully used to pulp banana fibres. It has proved to be an effective, alternative pulping agent as:
-
(a)
comparable lignin and hemicellulose removal, in comparison with the performance of the common sodium hydroxide, have been achieved;
-
(b)
pulped fibres having improved properties such as high crystallinity and low crystallite size have been obtained; and
-
(c)
high pulp yield has been attained, thereby proving the profitability of using wood ash solution for the pulping process.
Moreover, the investigation of the non-conventional factors namely, liquor ratio, input size and duration have shown that small fibre input size at a high liquor ratio of 40 for an extended period of 2 h are necessary to achieve maximum delignification.
Finally, the pulped fibres have depicted their suitability for use as ultrafiltration membranes by virtue of their aspect ratio, semi-hydrophilicity (due to presence of residual lignin) and projected high mechanical strength (due to high crystallinity and low crystallite size).
Data Availability
All data generated or analysed during this study are included in this published article and its supplementary file.
References
Mautner, A., Lee, K.Y., Lahtinen, P., Hakalahti, M., Tammelin, T., Li, K., Bismarck, A.: Nanopapers for organic solvent nanofiltration. Chem. Commun. 50, 5778–5781 (2014). https://doi.org/10.1039/c4cc00467a
Metreveli, G., Wågberg, L., Emmoth, E., Belák, S., Strømme, M., Mihranyan, A.: A size-exclusion nanocellulose filter paper for virus removal. Adv. Healthc. Mater. 3, 1546–1550 (2014). https://doi.org/10.1002/adhm.201300641
Asper, M., Hanrieder, T., Quellmalz, A., Mihranyan, A.: Removal of xenotropic murine leukemia virus by nanocellulose based filter paper. Biologicals 43, 452–456 (2015). https://doi.org/10.1016/j.biologicals.2015.08.001
Hakalahti, M., Salminen, A., Seppälä, J., Tammelin, T., Hänninen, T.: Effect of interfibrillar PVA bridging on water stability and mechanical properties of TEMPO/NaClO2 oxidized cellulosic nanofibril films. Carbohydr. Polym. 126, 78–82 (2015). https://doi.org/10.1016/j.carbpol.2015.03.007
Mautner, A., Lee, K.Y., Tammelin, T., Mathew, A.P., Nedoma, A.J., Li, K., Bismarck, A.: Cellulose nanopapers as tight aqueous ultra-filtration membranes. React. Funct. Polym. 86, 209–214 (2015). https://doi.org/10.1016/j.reactfunctpolym.2014.09.014
Quellmalz, A., Mihranyan, A.: Citric acid cross-linked nanocellulose-based paper for size-exclusion nanofiltration. ACS Biomater. Sci. Eng. 1, 271–276 (2015). https://doi.org/10.1021/ab500161x
Gustafsson, S., Mihranyan, A.: Strategies for tailoring the pore-size distribution of virus retention filter papers. ACS Appl. Mater. Interfaces. 8, 13759–13767 (2016). https://doi.org/10.1021/acsami.6b03093
Gustafsson, S., Lordat, P., Hanrieder, T., Asper, M., Schaefer, O., Mihranyan, A.: Mille-feuille paper: a novel type of filter architecture for advanced virus separation applications. Mater. Horizons. 3, 320–327 (2016). https://doi.org/10.1039/c6mh00090h
Mautner, A., Maples, H.A., Kobkeatthawin, T., Kokol, V., Karim, Z., Li, K., Bismarck, A.: Phosphorylated nanocellulose papers for copper adsorption from aqueous solutions. Int. J. Environ. Sci. Technol. 13, 1861–1872 (2016). https://doi.org/10.1007/s13762-016-1026-z
Manukyan, L., Li, P., Gustafsson, S., Mihranyan, A.: Growth media filtration using nanocellulose-based virus removal filter for upstream biopharmaceutical processing. J. Memb. Sci. 572, 464–474 (2019). https://doi.org/10.1016/j.memsci.2018.11.002
Wang, S., Gao, W., Chen, K., Xiang, Z., Zeng, J., Wang, B., Xu, J.: Deconstruction of cellulosic fibers to fibrils based on enzymatic pretreatment. Bioresour. Technol. 267, 426–430 (2018). https://doi.org/10.1016/j.biortech.2018.07.067
Mautner, A., Kobkeatthawin, T., Mayer, F., Plessl, C., Gorgieva, S., Kokol, V., Bismarck, A.: Rapid water softening with TEMPO-oxidized/phosphorylated nanopapers. Nanomaterials 9, 1–18 (2019). https://doi.org/10.3390/nano9020136
Helberg, R.M.L., Torstensen, J., Dai, Z., Janakiram, S., Chinga-Carrasco, G., Gregersen, Ø.W., Syverud, K., Deng, L.: Nanocomposite membranes with high-charge and size-screened phosphorylated nanocellulose fibrils for CO2 separation. Green Energy Environ. 6, 585–596 (2021). https://doi.org/10.1016/j.gee.2020.08.004
Koppolu, R., Blomquist, N., Dahlström, C., Toivakka, M.: High-throughput processing of nanographite-nanocellulose-based electrodes for flexible energy devices. Ind. Eng. Chem. Res. 59, 11232–11240 (2020). https://doi.org/10.1021/acs.iecr.0c01112
Pöhler, T., Mautner, A., Aguilar-Sanchez, A., Hansmann, B., Kunnari, V., Grönroos, A., Rissanen, V., Siqueira, G., Mathew, A.P., Tammelin, T.: Pilot-scale modification of polyethersulfone membrane with a size and charge selective nanocellulose layer. Sep. Purif. Technol. (2022). https://doi.org/10.1016/j.seppur.2021.120341
Gellerstedt, G., Ek, M., Henriksson, G.: Wood chemistry and wood biotechnology. De Gruyter, Sweden (2009)
Rahman, M.S., Hossain, M.E., Islam, M.R.: An environment-friendly alkaline solution for enhanced oil recovery. Pet. Sci. Technol. 26, 1596–1609 (2008). https://doi.org/10.1080/10916460701287557
Misra, M.K., Ragland, K.W.: Wood ash composition as a function of furnace temperature. Biomass Bioenerg. 4, 103–116 (1993)
Siddique, R.: Utilization of wood ash in concrete manufacturing. Resour. Conserv. Recycl. 67, 27–33 (2012). https://doi.org/10.1016/j.resconrec.2012.07.004
Sharma, M., Khan, A.A., Puri, S.K., Tuli, D.K.: Wood ash as a potential heterogeneous catalyst for biodiesel synthesis. Biomass Bioenerg. 41, 94–106 (2012). https://doi.org/10.1016/j.biombioe.2012.02.017
Eliche-Quesada, D., Felipe-Sesé, M.A., López-Pérez, J.A., Infantes-Molina, A.: Characterization and evaluation of rice husk ash and wood ash in sustainable clay matrix bricks. Ceram. Int. 43, 463–475 (2017). https://doi.org/10.1016/j.ceramint.2016.09.181
Legesse, A., Habtamu, A., Tegene, T.: Use of jatropha seed oil and alkali solution obtained from its ash for soap making: an environmentally friendly and cost effective approach. J. Appl. Sci. Environ. Manag. 24, 2005–2015 (2021). https://doi.org/10.4314/jasem.v24i12.1
Ekhuemelo, D.O., Oluwalana, S.A., A.A.C.: Potentials of Agricultural Waste and Grasses in Pulp and Papermaking. J. Res. For. Wildl. Environ. 4, (2012)
Du, C., Li, H., Liu, B., Chen, J., Hu, J., Zeng, J.: Effect of beating degree of fiber on the development of porosity in polyacrylonitrile-based activated carbon fiber paper. Diam. Relat. Mater. 128, 2–4 (2022). https://doi.org/10.1016/j.diamond.2022.109228
Sudhir, S., Sandeep, S.N., Zhe, Z., Arthur, J.R.Y.D.: Characterization of micro fibrillation process of cellulose and mercerized cellulose pulp. R. Soc. Chem. 5, 63111–63122 (2015)
Dinand, E., Vignon, M., Chanzy, H., Heux, L.: Mercerization of primary wall cellulose and its implication for the conversion of cellulose I → cellulose II. Cellulose 9, 7–18 (2002). https://doi.org/10.1023/A:1015877021688
Ramasawmy, H., Chummun, J., Emrith, P.D.: Slaked lime solution as a viable eco-friendly treatment to improve the tensile strength of maize stem fibers. J. Nat. Fibers. 00, 1–14 (2021). https://doi.org/10.1080/15440478.2021.1990177
Emrith, P.D., Ramasawmy, H., Surroop, D., Das, D.B.: Substantial size reduction of banana fibres and enhancement of fibre properties achieved through a single mechanical treatment. Waste Biomass Valoriz. (2022). https://doi.org/10.1007/s12649-022-01929-0
Fechter, C., Fischer, S., Reimann, F., Brelid, H., Heinze, T.: Influence of pulp characteristics on the properties of alkali cellulose. Cellulose 27, 7227–7241 (2020). https://doi.org/10.1007/s10570-020-03151-4
Popy, R.S., Nayeem, J., Arafat, K.M.Y., Rahman, M.M., Jahan, M.S.: Mild potassium hydroxide pulping of straw. Curr. Res. Green Sustain. Chem. 3, 100015 (2020). https://doi.org/10.1016/j.crgsc.2020.100015
Sanjay, M.R., Siengchin, S., Parameswaranpillai, J., Jawaid, M., Pruncu, C.I., Khan, A.: A comprehensive review of techniques for natural fibers as reinforcement in composites: preparation, processing and characterization. Carbohydr. Polym. 207, 108–121 (2019). https://doi.org/10.1016/j.carbpol.2018.11.083
Moghaddam, M.K., Mortazavi, S.M.: Physical and chemical properties of natural fibers extracted from Typha Australis leaves. J. Nat. Fibers. 13, 353–361 (2016). https://doi.org/10.1080/15440478.2015.1029199
Cordeiro, N., Belgacem, M.N., Torres, I.C., Moura, J.C.V.P.: Chemical composition and pulping of banana pseudo-stems. Ind. Crops Prod. 19, 147–154 (2004). https://doi.org/10.1016/j.indcrop.2003.09.001
Rongpipi, S., Ye, D., Gomez, E.D., Gomez, E.W.: Progress and opportunities in the characterization of cellulose—an important regulator of cell wall growth and mechanics. Front. Plant Sci. 9, 1–28 (2019). https://doi.org/10.3389/fpls.2018.01894
Khan, H., Yerramilli, A.S., D’Oliveira, A., Alford, T.L., Boffito, D.C., Patience, G.S.: Experimental methods in chemical engineering: X-ray diffraction spectroscopy—XRD. Can. J. Chem. Eng. 98, 1255–1266 (2020). https://doi.org/10.1002/cjce.23747
Park, C.W., Han, S.Y., Namgung, H.W., Seo, P.N., Lee, S.Y., Lee S.H.: Preparation and characterization of cellulose nanofibrils with varying chemical compositions. BioResources. 12, 5031–5044 (2017). https://doi.org/10.15376/biores.12.3.5031-5044
Moeller, T.: Chemistry: With Inorganic Qualitative Analysis, https://books.google.lk/books?hl=en&lr=&id=uyjjgw_EmXEC&oi=fnd&pg=PP1&dq=inorganic+chemistry+qualitative+analysis&ots=orPLb9ryya&sig=2aFus-PYZBgbwCvH09VBMmnfX70&redir_esc=y#v=onepage&q=inorganic chemistry qualitative analysis&f=false, (2012)
Ham, B.M., Aihui, M.: Analytical Chemistry: A Chemist and Laboratory Technician’s Toolkit 1st Edition, (2015)
Huynh, U.T.D., Lerbret, A., Neiers, F., Chambin, O., Assifaoui, A.: Binding of divalent cations to polygalacturonate: a mechanism driven by the hydration water. J. Phys. Chem. B. 120, 1021–1032 (2016). https://doi.org/10.1021/acs.jpcb.5b11010
Sedan, D., Pagnoux, C., Chotard, T., Smith, A., Lejolly, D., Gloaguen, V., Krausz, P.: Effect of calcium rich and alkaline solutions on the chemical behaviour of hemp fibres. J. Mater. Sci. 42, 9336–9342 (2007). https://doi.org/10.1007/s10853-007-1903-4
Rahman, N.S.A., Azahari, B.: Effect of calcium hydroxide filler on the mechanical properties of natural rubber latex films. J. Rubber Res. 15, 230–242 (2012)
Silva, F. de A., Filho, R.D.T., Filho, J. de A.M., Fairbairn, E. de M.R.: Physical and mechanical properties of durable sisal fiber-cement composites. Constr. Build. Mater. 24, 777–785 (2010). https://doi.org/10.1016/j.conbuildmat.2009.10.030
Ciobanu, M., Bobu, E., Ciolacu, F.: In-situ cellulose fibres loading with calcium carbonate precipitated by different methods. Cellul. Chem. Technol. 44, 379–387 (2010)
Le Troedec, M., Sedan, D., Peyratout, C., Bonnet, J.P., Smith, A., Guinebretiere, R., Gloaguen, V., Krausz, P.: Influence of various chemical treatments on the composition and structure of hemp fibres. Compos. Part A Appl. Sci. Manuf. 39, 514–522 (2008). https://doi.org/10.1016/j.compositesa.2007.12.001
Nelson, M.L., O’Connor, R.T.: Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 8, 1325–1341 (1964). https://doi.org/10.1002/app.1964.070080323
Åkerholm, M., Hinterstoisser, B., Salmén, L.: Characterization of the crystalline structure of cellulose using static and dynamic FT-IR spectroscopy. Carbohydr. Res. 339, 569–578 (2004). https://doi.org/10.1016/j.carres.2003.11.012
Liu, Y., Hu, T., Wu, Z., Zeng, G., Huang, D., Shen, Y., He, X., Lai, M., He, Y.: Study on biodegradation process of lignin by FTIR and DSC. Environ. Sci. Pollut. Res. 21, 14004–14013 (2014). https://doi.org/10.1007/s11356-014-3342-5
Alaejos, J., López, F., Eugenio, M.E., Tapias, R.: Soda-anthraquinone, kraft and organosolv pulping of holm oak trimmings. Bioresour. Technol. 97, 2110–2116 (2006). https://doi.org/10.1016/j.biortech.2005.09.021
Enqvist, E.: Impregnation, Vapor Phase and Methanol as Means of Intensifying the Softwood Kraft Pulping Process. (2006)
Paixão, S.M., Ladeira, S.A., Silva, T.P., Arez, B.F., Roseiro, J.C., Martins, M.L.L., Alves, L.: Sugarcane bagasse delignification with potassium hydroxide for enhanced enzymatic hydrolysis. R. Soc. Chem. (2015). https://doi.org/10.1039/x0xx00000x
Ramakrishna, G., Sundararajan, T.: Studies on the durability of natural fibres and the effect of corroded fibres on the strength of mortar. Cem. Concr. Compos. 27, 575–582 (2005). https://doi.org/10.1016/j.cemconcomp.2004.09.008
Carrillo, F., Colom, X., Suñol, J.J., Saurina, J.: Structural FTIR analysis and thermal characterisation of lyocell and viscose-type fibres. Eur. Polym. J. 40, 2229–2234 (2004). https://doi.org/10.1016/j.eurpolymj.2004.05.003
Jiménez, L., Ramos, E., Rodríguez, A., De La Torre, M.J., Ferrer, J.L.: Optimization of pulping conditions of abaca. An alternative raw material for producing cellulose pulp. Bioresour. Technol. 96, 977–983 (2005). https://doi.org/10.1016/j.biortech.2004.09.016
Lim, W.L., Gunny, A.A.N., Kasim, F.H., AlNashef, I.M., Arbain, D.: Alkaline deep eutectic solvent: a novel green solvent for lignocellulose pulping. Cellulose 26, 4085–4098 (2019). https://doi.org/10.1007/s10570-019-02346-8
French, A.D.: Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21, 885–896 (2014). https://doi.org/10.1007/s10570-013-0030-4
Chen, B., Sun, W., Wang, C., Guo, X.: Size-dependent impact of inorganic nanoparticles on sulfamethoxazole adsorption by carbon nanotubes. Chem. Eng. J. 316, 160–170 (2017). https://doi.org/10.1016/j.cej.2017.01.087
Augustine, R., Augustine, A., Kalarikkal, N., Thomas, S.: Fabrication and characterization of biosilver nanoparticles loaded calcium pectinate nano-micro dual-porous antibacterial wound dressings. Prog. Biomater. 5, 223–235 (2016). https://doi.org/10.1007/s40204-016-0060-8
Megra, M.B., Bachheti, R.K., Tadesse, M.G., Worku, L.A.: Evaluation of pulp and papermaking properties of melia azedarach. Forests (2022). https://doi.org/10.3390/f13020263
Li, Q., Wang, A., Ding, W., Zhang, Y.: Influencing factors for alkaline degradation of cellulose. BioResources. 12, 1263–1272 (2017). https://doi.org/10.15376/biores.12.1.1263-1272
Gu, Z.G., Li, D.J., Zheng, C., Kang, Y., Wöll, C., Zhang, J.: MOF-templated synthesis of ultrasmall photoluminescent carbon-nanodot arrays for optical applications. Angew. Chemie - Int. Ed. 56, 6853–6858 (2017). https://doi.org/10.1002/anie.201702162
French, A.D., Santiago Cintrón, M.: Cellulose polymorphy, crystallite size, and the Segal Crystallinity Index. Cellulose 20, 583–588 (2013). https://doi.org/10.1007/s10570-012-9833-y
Fink, H.P., Hofmann, D., Philipp, B.: Some aspects of lateral chain order in cellulosics from X-ray scattering. Cellulose 2, 51–70 (1995). https://doi.org/10.1007/BF00812772
Rodríguez, A., Moral, A., Serrano, L., Labidi, J., Jiménez, L.: Rice straw pulp obtained by using various methods. Bioresour. Technol. 99, 2881–2886 (2008). https://doi.org/10.1016/j.biortech.2007.06.003
Sharma, P.R., Sharma, S.K., Lindström, T., Hsiao, B.S.: Nanocellulose-enabled membranes for water purification: perspectives. Adv. Sustain. Syst. 4, 1–28 (2020). https://doi.org/10.1002/adsu.201900114
Nair, S.S., Yan, N.: Effect of high residual lignin on the thermal stability of nanofibrils and its enhanced mechanical performance in aqueous environments. Cellulose 22, 3137–3150 (2015). https://doi.org/10.1007/s10570-015-0737-5
Acknowledgements
The authors wish to thank Dr Keith Yendall in the Loughborough Materials Characterisation Centre, Loughborough University, UK for his kind support and assistance. The authors thank the Higher Education Commission, Mauritius for the financial support for the conduct of the research.
Funding
This study was financially supported by the Higher Education Commission (Mauritius).
Author information
Authors and Affiliations
Contributions
PDE: Conceptualization, Methodology, Investigation, Writing—Original Draft; HR: Writing—Review and Editing, Supervision; DS: Supervision, Writing—Review and Editing; DBD: Resources, Supervision, Writing—Review and Editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no competing interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emrith, P.D., Ramasawmy, H., Surroop, D. et al. Valorising Wood Ash as an Effective Green Alternative Pulping Agent for Achieving a High Yield of Banana Micro-Lignocellulose Fibres of Enhanced Crystallinity. Waste Biomass Valor 15, 821–840 (2024). https://doi.org/10.1007/s12649-023-02181-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02181-w