Abstract
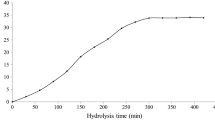
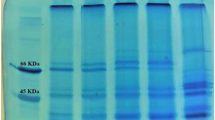
Feather is the most abundant keratinous material in nature. This by-product of animal origin has about 80–90% of crude protein. This work aimed to maximize the enzymatic hydrolysis of feather meal using the proteolytic enzyme from Bacillus sp. P45 and evaluate the antioxidant capacity of different fractions of the hydrolysate. Enzymatic hydrolysis was maximized using a central composite rotatable design (CCRD) 24, where the effects of the variables CaCl2 concentration (0–100 mmol/L), temperature (35–55 °C), enzyme/substrate ratio (600–6000 U/g-protein), and substrate protein concentration (10–40 g/L) were estimated on the responses degree of hydrolysis (DH) and protein recovery (PR). The highest values of DH and PR were obtained when the hydrolysis was performed with 50 mmol/L of CaCl2, 50 °C, 6000 U/g-protein, and substrate protein concentration of 10 g/L. Hydrolysate presented DH of 8.4% and PR of 37.5% at 6 h of reaction. The obtained peptides were separated by molecular weight through a sequential ultrafiltration process in the membranes of 10 and 3 kDa, and the antioxidant activities of the different fractions were analyzed. The fraction < 3 kDa showed a higher capacity to sequester radicals ABTS⋅+ (90.20 μmol TE/g) and peroxyl (1892.47 μmol TE/g). This study shows the potential of Bacillus sp. P45 in hydrolyzing feather meal, resulting in peptides with antioxidant activity.
Graphical Abstract




Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Tesfaye, T., Sithole, B., Ramjugernath, D.: Valorisation of chicken feathers: a review on recycling and recovery route-current status and future prospects. Clean Technol. Environ. Policy 19, 2363–2378 (2017). https://doi.org/10.1007/s10098-017-1443-9
Casadesús, M., Macanás, J., Colom, X., Cañavate, J., Álvarez, M.D., Garrido, N., Molins, G., Carrillo, F.: Effect of chemical treatments and additives on properties of chicken feathers thermoplastic biocomposites. J. Compos. Mater. 52, 3637–3653 (2018). https://doi.org/10.1177/0021998318766652
Callegaro, K., Welter, N., Daroit, D.J.: Feathers as bioresource: microbial conversion into bioactive protein hydrolysates. Process Biochem. 75, 1–9 (2018). https://doi.org/10.1016/j.procbio.2018.09.002
USDA: Livestock and Poultry: World Markets and Trade: Brazil Meat Exports Continue to Grow. (2021)
Tesfaye, T., Sithole, B., Ramjugernath, D., Chunilall, V.: Valorisation of chicken feathers: characterisation of chemical properties. Waste Manag. 68, 626–635 (2017). https://doi.org/10.1016/j.wasman.2017.06.050
Brandelli, A., Sala, L., Kalil, S.J.: Microbial enzymes for bioconversion of poultry waste into added-value products. Food Res. Int. 73, 3–12 (2015). https://doi.org/10.1016/j.foodres.2015.01.015
Tavano, O.L.: Protein hydrolysis using proteases: an important tool for food biotechnology. J. Mol. Catal. B 90, 1–11 (2013)
Cheison, S.C., Kulozik, U.: Impact of the environmental conditions and substrate pre-treatment on whey protein hydrolysis: a review. Crit. Rev. Food Sci. Nutr. 57, 418–453 (2017). https://doi.org/10.1080/10408398.2014.959115
Wasswa, J., Tang, J., Gu, X., Yuan, X.: Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin. Food Chem. 104, 1698–1704 (2007). https://doi.org/10.1016/j.foodchem.2007.03.044
Jemil, I., Abdelhedi, O., Nasri, R., Mora, L., Jridi, M., Aristoy, M.C., Toldrá, F., Nasri, M.: Novel bioactive peptides from enzymatic hydrolysate of Sardinelle (Sardinella aurita) muscle proteins hydrolysed by Bacillus subtilis A26 proteases. Food Res. Int. 100, 121–133 (2017). https://doi.org/10.1016/j.foodres.2017.06.018
Spellman, D., O’Cuinn, G., FitzGerald, R.J.: Bitterness in Bacillus proteinase hydrolysates of whey proteins. Food Chem. 114, 440–446 (2009). https://doi.org/10.1016/j.foodchem.2008.09.067
Razzaq, A., Shamsi, S., Ali, A., Ali, Q., Sajjad, M., Malik, A., Ashraf, M.: Microbial proteases applications. Front. Bioeng. Biotechnol. 7, 1–20 (2019). https://doi.org/10.3389/fbioe.2019.00110
Rao, M., Tanskale, A., Ghatge, M., Deshpande, V.: Molecular and biotechnological aspects of microbial proteases. Microbiol. Mol. Biol. Rev. 62, 597–635 (1998). https://doi.org/10.1016/S0168-6445(99)00006-6
Daroit, D.J., Corrêa, A.P.F., Brandelli, A.: Keratinolytic potential of a novel Bacillus sp P45 isolated from the Amazon basin fish Piaractus mesopotamicus. Int. Biodeterior. Biodegrad. 63, 358–363 (2009). https://doi.org/10.1016/j.ibiod.2008.11.008
Olagunju, A.I., Omoba, O.S., Enujiugha, V.N., Alashi, A.M., Aluko, R.E.: Pigeon pea enzymatic protein hydrolysates and ultrafiltration peptide fractions as potential sources of antioxidant peptides: an in vitro study. LWT Food Sci. Technol. 97, 269–278 (2018). https://doi.org/10.1016/j.lwt.2018.07.003
Zhang, M., Mu, T.H.: Identification and characterization of antioxidant peptides from sweet potato protein hydrolysates by Alcalase under high hydrostatic pressure. Innov. Food Sci. Emerg. Technol. 43, 92–101 (2017). https://doi.org/10.1016/j.ifset.2017.08.001
Wang, P., Lin, Y., Wu, H., Lin, J., Chen, Y., Hamzah, S.S., Zeng, H., Zhang, Y., Hu, J.: Preparation of antioxidant peptides from hairtail surimi using hydrolysis and evaluation of its antioxidant stability. Food Sci. Technol. 2061, 1–11 (2020). https://doi.org/10.1590/fst.23719
Gómez-Sampedro, L.J., Zapata-Montoya, J.E.: Obtaining of antioxidant peptide from bovine plasma hydrolysates and effect of the degree of hydrolysis on antioxidant capacity. Rev. Mex. Ing. Quim. 15, 101–109 (2016)
Zhang, J., Wang, J., Zhao, Y., Li, J., Liu, Y.: Study on the interaction between calcium ions and alkaline protease of Bacillus. Int. J. Biol. Macromol. 124, 121–130 (2019). https://doi.org/10.1016/j.ijbiomac.2018.11.198
Daroit, D.J., Sant’Anna, V., Brandelli, A.: Kinetic stability modelling of keratinolytic protease P45: influence of temperature and metal ions. Appl. Biochem. Biotechnol. 165, 1740–1753 (2011). https://doi.org/10.1007/s12010-011-9391-z
Bertsch, A., Coello, N.: A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Biores. Technol. 96, 1703–1708 (2005). https://doi.org/10.1016/j.biortech.2004.12.026
Daroit, D.J., Corrêa, A.P.F., Brandelli, A.: Production of keratinolytic proteases through bioconversion of feather meal by the Amazonian bacterium Bacillus sp. P45. Int. Biodeterior. Biodegrad. 65, 45–51 (2011). https://doi.org/10.1016/j.ibiod.2010.04.014
Adler-Nissen, J., Eriksen, S., Olsen, H.S.: Improvement of the functionality of vegetable proteins by controlled enzymatic hydrolysis. Qualitas Plantarum Plant Foods Hum. Nutr. 32, 411–423 (1983). https://doi.org/10.1007/BF01091198
Zaraî Jaouadi, N., Jaouadi, B., Aghajari, N., Bejar, S.: The overexpression of the SAPB of Bacillus pumilus CBS and mutated sapB-L31I/T33S/N99Y alkaline proteases in Bacillus subtilis DB430: new attractive properties for the mutant enzyme. Biores. Technol. 105, 142–151 (2012). https://doi.org/10.1016/j.biortech.2011.11.115
Kirk, P.L.: Kjeldahl method for total nitrogen. Anal. Chem. 22, 354–358 (1950). https://doi.org/10.1021/ac60038a038
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J.: Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193, 265–275 (1951). https://doi.org/10.1007/978-94-007-0753-5_100521
Re, R., Pelligrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-Evans, C.: Antioxidant activity applying an improved ABTS radical cation decolorization assay. J. Food Sci. Technol. 26, 1231–1237 (1999). https://doi.org/10.1016/j.indcrop.2017.04.056
Rodrigues, E., Mariutti, L.R.B., Faria, A.F., Mercadante, A.Z.: Microcapsules containing antioxidant molecules as scavengers of reactive oxygen and nitrogen species. Food Chem. 134, 704–711 (2012). https://doi.org/10.1016/j.foodchem.2012.02.163
Cao, Z.J., Zhang, Q., Wei, D.K., Chen, L., Wang, J., Zhang, X.Q., Zhou, M.H.: Characterization of a novel Stenotrophomonas isolate with high keratinase activity and purification of the enzyme. J. Ind. Microbiol. Biotechnol. 36, 181–188 (2009). https://doi.org/10.1007/s10295-008-0469-8
Smith, C.A., Toogood, H.S., Baker, H.M., Daniel, R.M., Baker, E.N.: Calcium-mediated thermostability in the subtilisin superfamily: the crystal structure of Bacillus Ak1 protease at 18 Å resolution. J. Mol. Biol. 294, 1027–1040 (1999). https://doi.org/10.1006/jmbi.1999.3291
Daroit, D.J., Corrêa, A.P.F., Segalin, J., Brandelli, A.: Characterization of a keratinolytic protease produced by the feather-degrading Amazonian bacterium Bacillus sp. P45. Biocatal. Biotransform. 28, 370–379 (2010). https://doi.org/10.3109/10242422.2010.532549
Fisher, K.E., Ruan, B., Alexander, P.A., Wang, L., Bryan, P.N.: Mechanism of the kinetically-controlled folding reaction of subtilisin. Biochemistry 46, 640–651 (2007). https://doi.org/10.1021/bi061600z
Zhang, H., Yu, L., Yang, Q., Sun, J., Bi, J., Liu, S., Zhang, C., Tang, L.: Optimization of a microwave-coupled enzymatic digestion process to prepare peanut peptides. Molecules 17, 5661–5674 (2012). https://doi.org/10.3390/molecules17055661
Zhou, D., Qin, L., Zhu, B., Li, D., Yang, J., Dong, X., Murata, Y.: Optimisation of hydrolysis of purple sea urchin (Strongylocentrotus nudus) gonad by response surface methodology and evaluation of in vitro antioxidant activity of the hydrolysate. J. Sci. Food Agric. 92, 1694–1701 (2012). https://doi.org/10.1002/jsfa.5534
Chen, X., Luo, Y., Qi, B., Luo, J., Wan, Y.: Improving the hydrolysis efficiency of soy sauce residue using ultrasonic probe-assisted enzymolysis technology. Ultrason. Sonochem. 35, 351–358 (2017). https://doi.org/10.1016/j.ultsonch.2016.10.013
Kurozawa, L.E., Park, K.J., Hubinger, M.D.: Optimization of the enzymatic hydrolysis of chicken meat using response surface methodology. J. Food Sci. 73, 405–412 (2008). https://doi.org/10.1111/j.1750-3841.2008.00765.x
Eijsink, V.G.H., Matthews, B.W., Vriend, G.: The role of calcium ions in the stability and instability of a thermolysin-like protease. Protein Sci. 20, 1346–1355 (2011). https://doi.org/10.1002/pro.670
Holanda, H.D., Netto, F.M.: Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis. J. Food Sci. 71, 298–303 (2006). https://doi.org/10.1111/j.1750-3841.2006.00040.x
Bhaskar, N., Benila, T., Radha, C., Lalitha, R.G.: Optimization of enzymatic hydrolysis of visceral waste proteins of Catla (Catla catla) for preparing protein hydrolysate using a commercial protease. Biores. Technol. 99, 335–343 (2008). https://doi.org/10.1016/j.biortech.2006.12.015
Sarmadi, B.H., Ismail, A.: Antioxidative peptides from food proteins: a review. Peptides 31, 1949–1956 (2010). https://doi.org/10.1016/j.peptides.2010.06.020
Sbroggio, M., Montilha, M., Figueiredo, V., Georgetti, S., Kurozawa, L.: Influence of the degree of hydrolysis and type of enzyme on antioxidant activity of okara protein hydrolysates. Food Sci. Technol. 36, 375–381 (2016). https://doi.org/10.1590/1678-457X.000216
Ngoh, Y.Y., Gan, C.Y.: Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 190, 331–337 (2016). https://doi.org/10.1016/j.foodchem.2015.05.120
Guo, P., Qi, Y., Zhu, C., Wang, Q.: Purification and identification of antioxidant peptides from Chinese cherry (Prunus pseudocerasus Lindl.) seeds. J. Funct. Foods. 19, 394–403 (2015). https://doi.org/10.1016/j.jff.2015.09.003
Funding
This study was financed by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) grant code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) Grants 423285/2018-1, 304857/2018-1 and 308880/2021-8, and São Paulo Research Foundation (FAPESP, Brazil) Grant 01550-8/2018. The authors are also grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à pesquisa do Estado do Rio Grande do Sul (FAPERGS) for the scholarships.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, analysis, investigation, writing- original draft, review and editing and visualization were performed by ICC. Methodology, supervision and writing—review and editing were performed by ARCB. Methodology and writing—review and editing were performed by AB. Methodology and writing—review and editing and supervision were performed by LS. Conceptualization, writing—review and editing, methodology, supervision, project administration and funding acquisition were performed by SJK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Cunha, I.C., Brandelli, A., Braga, A.R.C. et al. Feather Meal as a Source of Peptides with Antioxidant Activity from Enzymatic Hydrolysis. Waste Biomass Valor 14, 421–430 (2023). https://doi.org/10.1007/s12649-022-01886-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01886-8