Abstract
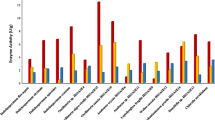
The genus Nostoc is a valuable source of a wide spectrum of natural products, which have many different biological activities such as antioxidant, anticancer, anti-HIV, antimalarial, antifungal and antimicrobial drugs. The current work was aimed to evaluate the biological activities of Nostoc muscorum microalga cultivated on different waste water samples (domestic, agricultural and industrial waste water). The algae was cultivated in different kinds of waste water; sterilized BG11 media, non-sterilized treated sewage waste water media (NS-TSW), sterilized treated sewage waste water media (S-TSW), 50% BG11 + 50% sterilized TSW media (50% S-TSW), non-sterilized industrial waste water media (NS-IW), sterilized industrial waste water media (S-IW), 50% BG11 + 50% sterilized industrial waste water media (50% S-IW), non-sterilized agriculture waste water media (NS-AW), sterilized agriculture waste water media (S-AW), and 50% BG11 + 50% sterilized AW media (50% S-AW). The experiment was conducted in triplicate and cultures were incubated at 25 ± 1 °C under continuous shaking (150 rpm) and illumination (2000 lx) for 15 days. The pigments content and biological activity include antioxidant (using diphenyl picryl hydrazyl and 2,2-azino-bis ethylbenzthiazoline-6-sulfonic acid radical methods), antiviral activity against Herpes simplex virus and the cytotoxicity for aqueous extracts in vivo and in vitro assays were determined. The given results reported that use of waste water as media for cultivation of microalgae is a suitable and inexpensive method when compared with ordinary cultivation methods. Also We can conclude that the algal species has high ability to produce active ingredients used as antioxidants and antivirals especially from algae cultivated in S-TSW, 50% S-TSW, NS-AW and 50% S-AW without any toxicity in mice.




Similar content being viewed by others
Abbreviations
- ABTS:
-
2,2-Azino-bis ethylbenzthiazoline-6-sulfonic acid
- AW:
-
Agricultural waste water
- ALT:
-
Alanine aminotransferase
- APC:
-
Allophycocyanin
- AST:
-
Aspartate aminotransferase
- BHA:
-
Butylated hydroxyl anisol
- BUN:
-
Blood urea nitrogen
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- DPPH:
-
Diphenyl picryl hydrazyl
- HDL:
-
High density lipoprotein
- GLU:
-
Glucose
- IW:
-
Industrial waste water
- LDL:
-
Low density lipoprotein
- PE:
-
Phycoerythrin
- TSW:
-
Treated sewage waste water
References
Oudra, B., Andaloussi, M.E., Vasconcelos, V.M.: Identification and quantification of microcystins from a Nostoc muscorum bloom occurring in Oukaïmeden River (High-Atlas mountains of Marrakech, Morocco). Environ. Monit. Assess. 149, 437–444 (2009)
Mostafa, S.M.S., Shalaby, E.A., Mahmoud, G.I.: Cultivating microalgae in domestic waste water for biodiesel production. Not. Sci. Biol. 4(1), 56–65 (2012)
Noue, J.D., Laliberte, G., Proulx, D.: Algae and waste water. J. Appl. Phycol. 4(3), 247–254 (1992)
Shalaby, E.A., Shanab, S.M.: Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Mar. Sci. 42(5), 556–564 (2013)
Nagasathya, A., Thajuddin, N.: Decolorization of paper mill effluent using hypersaline cyanobacteria. Res. Environ. Sci. 2(5), 408–414 (2008)
Amirjani, M.R.: Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int. J. Bot. 7, 73–81 (2011)
Karthikeyan, C.V., Gopalaswamy, G.: Studies on acid stress tolerant proteins of cyanobacterium. Int. J. Biol. Chem. 3, 1 (2009)
Kumar, A.S., Miao, Z.H., Wyatt, S.K.: Influence of nutrient loads, feeding frequency and inoculum source on growth of Chlorella vulgaris in digested piggery effluent culture medium. Biores. Technol. 101(15), 6012–6018 (2010)
Singh, N., Rajini, P.S.: Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 85, 611–616 (2004)
Pulz, O., Gross, W.: Valuable products from biotechnology of microalgae. J. Appl. Microbiol. Biotechnol. 65, 635–648 (2004)
Shalaby, E.A.: (2004). Chemical and biological studies on spirulina species. M Sc. Thesis, Department of Biochemistry, Faculty of Agriculture, Cairo University
Rippka, R., Deruelles, J., Waterbury, J., Herdman, M., Stanier, R.: Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111, 1–61 (1979)
APHA (1998). Standard methods for the examination of water and waste water, 20 edn. American Public Health Association, Washington, DC.
Bryant, D.A.: Phycoerythrin and phycocyanin properties and occurance in cyanobacteria. J. Gen. Microbiol. 128, 835–844 (1979)
Rossenthaler, L.:The Chemical Investigation of Plants. Translated into English by Sudhamoy Ghosh from the Third German edition. Bell and Sons. Ltd, London (1930)
Burits, M., Bucar, F.: Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 14, 323–328 (2000)
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-Evans, C.: Antioxidant activity applying improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26, 101–105 (1999)
KaviarasanS.,G.H.Naik,R.Gangabhagirathi,C.V.Anuradha, K.I.Priyadarsini: In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum-graecum) seeds. Food Chem.103, 31–37 (2007)
Silva, O., Barbose, S., Diniz, A., Valdeira, M., Gomes, E.: Plant extracts antiviral activity against Herpes simplex virus type 1 and African swine fever virus. Int. J. Pharm. 35, 12–16 (1997)
Mossman, T.: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 65, 55–63 (1983)
Bajpai, R., Sharma, N.K., Rai, A.K.: Hepatosplenomegaly and phytotoxicity of a planktonic cyanobacterium Nostoc sp. BHU001 isolated from agricultural pond. World J. Micro. Biotech. 25(11), 1995–2003 (2009)
Repavich, W.M., Sonzogni, W.C., Standridge, J.H., Wedepohl, R.E., Meisner, L.F.: Cyanobacteria (blue-green algae) in Wisconsin waters: acute and chronic toxicity. Water Res. 24(2), 225–231 (1990)
Erker, F., Slauohte, J., Bass, E.L., Pnvion, J., Wutoh, J.: (1985). Acute toxic effects in mice of an extract from the marine algae Gonyaulax monila ta. Toxicon. 23(5), 761–767
Sotero-Santos, R.B., Carvalho, E.G., Dellamano-Oliveira, M.J.: Occurrence and toxicity of an Anabaena bloom in a tropical reservoir (Southeast Brazil). Harmful Algae. 7, 590–598 (2008)
Carson, F.L., Hladik, C.: Histotechnology, A Self-Instructional Text, 3rd edn. American Society of Clinical Pathology Press, Oxford. pp 400, (2009)
Sokal, R.R., Rohlf, F.J., 1981. Biometry, 2nd edn. W. H. Freeman & Co., San Francisco, pp. 241–242
El-Sheekh, M.M., El-Shouny, W.A., Osman, E.H., El-Gammal, E.W.: Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial waste water effluents. Environ. Toxicol. Pharmacol. 19(2), 357–365 (2005)
Wang, L., Min, M., Li, Y., Chen, P., Chen, Y., Liu, Y., Wang, Y., Ruan, R.: Cultivation of green algae Chlorella sp. in different waste water from municipal waste water treatment plant. J. Appl. Biochem. Biotechnol. 162(4), 174–186 (2009)
Sallal, A.K.J.: Growth of cyanobacteria on sewage effluents. Microbios. 46, 121–129 (1986)
Vasconcelos, V.M., Pereira, E.: Cyanobacteria diversity and toxicity in a waste water treatment plant (Portugal). J. Water Res. 35, 1354–1357 (2001)
Glazer, A.N.: Phycobiliproteins a family of valuable widely used fluorophores. J. Appl. Phycol. 6, 105–112 (1994)
Shalaby, E.A., Shanab, S.M.M, Singh, V.: Salt stress enhancement of antioxidant and antiviral efficiency of Spirulina platensis. J. Med. Plant Res. 4(24), 2622–2632 (2010)
Shalaby, E.A., Nasr, N.F., Sherief, S.M.: An in vitro study of the antimicrobial and antioxidant efficacy of some nature essential oils. J. Med. Plant Res. 5(6), 922–931 (2011)
Shalaby, E.A.: Algae as promising organisms for environment and health. Plant Signal. Behav. 6(9), 1338–1350 (2011)
Shanab, S.M.M., Mostafa, S.S.M., Shalaby, E.A., Mahmoud, G.I.: Aqueous extracts of microalgae exhibit antioxidant and anticancer activities. Asian Pac. J. Trop. Biomed. 2(8), 608–615 (2012)
Fooa, S.C., Yusoff, F.Md., Ismail, M., Basri, M., Yaua, S.K., Khonga, N.M.H., Chana, K.W., Ebrahimi, M.: Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. J. Biotechnol. 241, 175–183 (2017)
Sato, Y., Itagaki, S., Kurokawa, T., Ogura, J., Kobayashi, M., Hirano, T., Sugawara, M., Iseki, K.: In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharmaceut. 403, 136–138 (2011)
Romeilah, R.M., Fayed, S.A., Mahmoud, G.I.: Chemical compositions, antiviral and antioxidant activities of seven essential oils. J. Appl. Sci. Res. 6, 50–62 (2010)
Qiu, D., Guo, J., Yu, H., Yang, S., Li, X., Zhang, Y., Sun, J., Cong, J., He, S., Wei, D., Qin, J.: Antioxidant phenolic compounds isolated from wild Pyrus ussuriensis Maxim. fruit peels and leaves. Food Chem. 241(15), 182–187 (2018)
Erker, F., Slauohte., J., Bass, E.L., Pnvion, J., Wutoh, J.: Acute toxic effects in mice of an extract from the marine algae Gonyaulax monila ta. Toxicity. 23(5), 761–767 (1985)
Carmichael, W.W.: Algal toxins. In: Callow R. Advances in Botanical Research, vol. 12, p. 47101. Academic Press, London (1986)
Machleder, D., Ivandic, B., Welch, C., Castellani, L., Reue, K.: Complex genetic control of HDL levels in mice in response to an atherogenic diet. Coordinate regulation of HDL levels and bile acid metabolism. J. Clin. Invest. 99, 1406–1419 (1997)
He, M., Su, H., Gao, W., Johansson, S.M., Liu, Q., Wu, X., Young, A.A., Bartfai, T., Wang, M.W., Liao, J.: Reversal of obesity and insulin resistance by a non-peptidic glucagon-like peptide-1 receptor agonist in diet-induced obese mice. PLoS ONE. 5(12), e14205 (2010)
Serfilippi, L.M., Stackhouse, P.D., Russell, B., Spainhour, C.B.: Serum clinical chemistry and hematology reference values in outbred stocks of albino mice from three commonly used vendors and two inbred strains of albino mice. J. Am. Assoc. Lab. Animal Sci. 42(3), 46–52 (2003)
Fernández, I., Peña, A., Teso, N.D., Pérez, V., Rodríguez-Cuesta, J.: Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J. Am. Assoc. Lab. Anim. Sci. 49(2), 202–206 (2010)
Anon.: The National Cholesterol Education Program. J. American Medi. Assoc. 280(24), 2099–2104 (1998)
Anon.: Normal Values In The Mouse. Iowa State University of Science and Technology (2012). http://www.lar.iastate.edu/index.php?option=com_content&view=article&id=113&Itemid=136
Acknowledgements
The authors are thankful to Faculty of Agriculture in University of Cairo and University of Tanta for providing the facilities.
Funding
This work was partially supported by a grant from the Science and Technology Development Fund (STDF-Project ID: 312), Cairo, Egypt.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: MBA, EAS, MAM. Performed the experiments: IAS, EAS, GIM Collection of data: IAS, MBA, EAS, MAM. Analyzed the data: MBA, EAS, IAS, HAE. DAL Contributed reagents/materials/analysis tools: MBA, IAS, EAS, GIM. Wrote the paper: EAS, HAE, DAL. Revising of manuscript: EAS, HAE, DAL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Shalaby, E.A., Atta, M.B., Sleem, I.A. et al. Cytotoxicity, Antioxidant and Antiviral Potential of Aqueous Extract from Nostoc muscorum Cultivated in Various Inexpensive Media. Waste Biomass Valor 10, 1419–1431 (2019). https://doi.org/10.1007/s12649-017-0188-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-017-0188-3