Abstract
Biodiesel is an attractive alternative fuel for diesel engines produced from vegetable oils or animal fats by transesterification in the presence of a catalyst. The feedstocks used in this experiment for the production of biodiesel were waste frying oil (WFME) waste canola oil (WCME), and their different percentages are referred to as 25-WFME, 50-WFME and 75-WFME. The samples are sourced from Northern Cyprus. Also, biodiesel was produced at Mechanical Engineering research laboratory. The purpose of this study is to investigate the effect of the temperature on the kinematic, dynamic viscosity and density of the biodiesel samples. Also, this paper examines the cold flow properties as well as the kinematic viscosity, cloud point (CP) and pour point (PP) of the produced biodiesel. The density, kinematic viscosity, CP and PP measurements were made according to ASTM standards. The properties of the produced biodiesel such as viscosity and density were measured within temperature ranges 20–270 °C in steps of 10 °C. The kinematic viscosity, CP and PP were measured within temperature range −10–20 °C. In this study, five general correlations were presented for estimating the density and kinematic viscosity of the blends at several temperatures. The experimental investigation showed that CP and PP of WCME increased with an increase in the concentration of WFME. Furthermore, empirical equations for predicting the viscosity, density, CP and PP for biodiesel samples give values in good agreement with experiments. The results obtained show that with the increase of the temperature, kinematic viscosity, dynamic viscosity and density decreased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biodiesel is a clean-burning, renewable fuel, which is defined as the monoalkyl esters of long fatty acids [1–4]. In order to decrease the price of biodiesel, attention has recently been focused on non-edible oils, especially on waste vegetable oils, which are renewable and easily available. Many researchers have investigated the transesterification of waste vegetable oils for the synthesis of biodiesel [5–10]. Transesterification does not change the fatty acid composition of the feedstock. The composition plays an important role to determine some of the main parameters of the biodiesel such as viscosity and cold flow properties. Chain lengths, branching of the chain and degree of saturation generally affect the physical and fuel properties of a fatty acid methyl ester [11, 12]. A change in the nature of the fatty acid profile in the biodiesel leads to overall changes in its fuel properties [13, 14].
One of the most important properties of biodiesel is viscosity, because it affects the fuel injection equipment [15–17], especially in cold weather, since viscosity increases as temperature rises [18]. Biodiesel viscosity is usually higher than that of diesel, and this higher viscosity level result in longer liquid penetration times and worse atomization [17, 19] compared to the case with diesel fuel. Previous studies revealed that the density and viscosity of biodiesel depend on its composition and the temperature [20–22]. It was observed that both viscosity and density decrease as the temperature increases.
The effects of main transesterification reaction parameters including the catalyst amount, reaction temperature, reaction time, alcohol/oil molar ratio, on biodiesel properties were investigated by various researchers using different optimization methods such as Response Surface Methodology [23–26]. It has been reported that, an increase in the reaction temperature and alcohol/oil molar ratio lowered the biodiesel yield due to the evaporation of alcohol. Moreover, other researchers have investigated the effect of alcohols on transesterification reaction for the production of biodiesel [27]. It has been concluded that higher alcohols have lesser reactivity so need more catalyst and higher temperature to speed up the reaction.
Several cold flow properties of the diesel and biodiesel fuel are generally used to classify cold weather performance, in terms of the cloud point (CP) and pour point (PP). Researchers have applied various experimental techniques to investigate the cold flow behavior of biodiesel (CP and PP) production from various feedstocks [28–32]. It was revealed that at the temperatures approaching the beginning of crystal formation, the viscosity rapidly increases, and the viscosity of the blend changes of between biodiesel and the diesel.
Some research has been conducted to identify the effect of higher alcohols on biodiesel properties and also to study how cold flow properties can be improved [33]. The most popular higher alcohols are methanol and ethanol, but additional higher alcohols such as 1-propanol, n-butanol butan-2-ol, and iso-propanol are also used. It was obtained that these branched-chain esters reduced the biodiesel crystallization temperature [33].
Several studies have been carried out that focused on replacing diesel fuel with biodiesel, which can be used alone or in blends with diesel fuel [34, 35]. For this reason, in recent years there has been a growing interest on biodiesel production from various vegetable oils in order to widen the available alternatives to diesel fuel.
In this study, biodiesel from waste frying oil and waste canola oil were prepared through transesterification reaction. The effects of temperature (from 20 to 270 °C) on kinematic viscosity, dynamic viscosity, and density of waste oil based biodiesel were examined and compared with those in the standards. Other properties such as Cloud Point, Pour Point as well as kinematic energy were also evaluated from the onset of crystallization to 20 °C. Furthermore, mathematical expressions of temperature dependence of these physical parameters are given to characterize the samples studied. The target of this study is to examine their feasibility for biodiesel production from waste oil in order to promote an economical and environmentally friendly process for biodiesel production. This is the first study to conduct such an investigation in Northern Cyprus.
Methodology
Biodiesel Samples
Waste frying oil samples were collected from Northern Cyprus and filtered to remove existing inorganic residues. The biodiesel productions from waste oils have been studied in laboratory environment. The similarities in the composition of vegetable oils/animal fats and petroleum derived diesel sustain their conversion to biodiesel [36–38]. Five different biodiesel samples sourced via the transesterification technique were employed, which remains one of the most promising methods [39]. The process transpires in a sequence of three consecutive reversible reactions: triglyceride gets converted stepwise into diglyceride, monoglyceride, and finally, glycerol as a result of which one mole of alkyl ester surfaces in each step [40, 41]. In order to avoid the negative impacts of biofuels on food prices and supplies [42], the transesterification processes in the present study expend waste frying oil and waste canola oil. In this context, waste frying oil refers to a mixture of used frying oil sourced from different restaurants as produced from different feedstock, and waste canola oil refers to used oil from pure canola vegetable oil.
Henceforth, WFME signifies methyl esters of waste frying oil. WCME represents methyl esters of waste canola oil and their different mixing percentages are referred to as 25-WFME, 50-WFME, and 75-WFME, where the number in the prefix indicates the percentage of WFME present in the mixture. Table 1 compares the fatty acid methyl ester composition of WFME and WCME. Also, the composition of each fatty acid present in the mixtures was calculated proportionally according to the required percentages. The fatty acid methyl ester of biodiesel produced from the waste frying oil and canola oil is compared with the available published data in the literature [43–53]. The most common fatty acid components of biodiesel feedstock in the literature were compared with current work, namely, C12:0, C14:0, C16:0, C18:0, C18:1, C18:2, C18:3, C20:0 and C20:1. It is found that the fatty acid composition of the current work was within the range of maximum and minimum of eight fatty acids obtained from the literature and were in good agreement with the published data as shown in Table 1. All samples were sourced from North Cyprus, with the climatic condition tagged as subtropical climate-Mediterranean, according to Koppen climate classification [54]. Calsisol, Leptosol and Vertisol exist as the major soil groups in this area as per Harmonized World Soil Database [55].
Biodiesel Analysis
Biodiesel standards are in place to ensure that only high-quality biodiesel reaches the marketplace. The two most important fuel standards are ASTM D6751 [56] in the United States and EN 14214 (European Committee for Standardization (CEN) [57] in the European Union. Table 2 summarizes the limit values of density and kinematic viscosity for biodiesel and biodiesel petrodiesel blend (B6–B20) fuel, ASTM D7467 [58], ASTMD975 [59], EN 590 [60], ASTM D396 [61] and EN 14213 [62]. In the cases of ASTM D7467, D975, and D396, the biodiesel component must satisfy the requirements of ASTM D6751 before inclusion in the respective fuels. Correspondingly, in the European Union, biodiesel must satisfy EN 14214 before inclusion into petrodiesel, as mandated by EN 590.
Experimental Setups
The biodiesel samples were analyzed to determine their viscosity, density, and cold flow properties (CP and PP). The effect of temperature on the biodiesel properties including kinematic viscosity, dynamic viscosity, density were tested within the temperature range of 20–270 °C to consider the effect of the injector and fuel supply line temperature. The cold flow properties increased with a higher viscosity values. In addition, high viscosity fuels will take more time to mix with the air inside the engine reducing the quality of the vaporization and atomization of the fuel. To reduce the impact of viscosity, the blending method has a positive effect on diesel engine performance as it improves lubricity [63–67]. Therefore, the temperature effect on cold flow properties and kinematic viscosity were investigated over the temperature range from the onset of crystallization until 20 °C.
Kinematic Viscosity Setup
From 20 to 270 °C
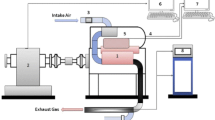
Ubbelohde viscometer (ASTM) was used to measure the kinematic viscosity because of its well-known application and accuracy. It enables transparent and high temperature measurement [68]. To ensure precise and stable temperature control during measurements, a digital temperature controller resistance was used to measure the temperature. A uniform temperature inside the silicon oil bath was attained. However, the mixer enabled the regulation of the temperature of a heated oil bath containing the viscometer by means of an electric heater. The temperature of the oil bath was varied from 20 to 270 °C. Each sample was tested four times, and the average viscosity was calculated. Figure 1 shows the experimental setup used to determine the temperature dependence of density and viscosity of the samples analyzed. In order to precisely determine the relationship between the time of flow and the kinematic viscosity for the three viscometers used, calibration of the instrument is necessary. The calibration was done by the manufacturer, SI Analytics GmbH, Mainz according to ASTM D 2525/D 446 and ISO/DIS 3105. The instrument constant, K, [(mm2/s)/s] was determined and given as in Table 3. The calibration constant can be used up to the temperature of 270 °C, and the influence of the temperature on the capillary constant due to thermal expansion of the Duran glass is negligible [69]. For absolute measurement, the corrected flow time multiplied by the viscometer constant K directly gives the kinematic viscosity (mm2/s) as given in Eq. (1).
where ν, K, t, and y represent the kinematic viscosity, the calibration constant, measured time of flow and kinetic energy correction [70] (HC, as given by the manufacturer) respectively.
From −10 to 20 °C
Figure 2 shows the experimental setup used to measure the kinematic viscosity of vegetable oils in the temperature range −10 to 20 °C. The experimental setup consists of a cooling bath (glass aquarium), a compressor, a mixer, and a thermostat. Alcohol (ethanol) is the simplest and cheapest cooling bath. To obtain a uniform temperature distribution within cooling bath, the cooling bath is equipped with a mixer to circulate the alcohol. The bath temperature was controlled using a thermostat, by automatically starting up and shutting down the compressor. A coil connected to a compressor cools down the liquid bath, and the compressor is cooled down by a radiator as shown in Fig. 2. The cooling bath was thermally isolated from the rest of its surroundings by a 3 cm thick Styrofoam layer.
Density Setup
The density measurement was made according to ASTM standard D854. The density of the biodiesel was measured using a pycnometer with a bulb capacity of 25 ml. However, all density measurements were performed in triplicate with the pycnometer. The weighting was done by using a high precision AND an electronic balance (model HR 300, Japan) with a precision of ± 0.1 mg. The density values of the samples were measured for temperatures between 20 to 270 °C. The experimental setup of measuring the density of biodiesel samples is shown in Fig. 1.
Cloud Point and Pour Point Setup
The biodiesel samples were tested as per American standard test method for Cloud Point and Pour Point, ASTMD2500, and ASTMD97, respectively. The setup mainly consisted of a cooling bath, glass test jar, thermocouples, multi-thermometer, and a regulator as depicted in Fig. 3. A glass test jar was placed in an aluminum cylinder inside the cooling bath liquid. The glass test jar was isolated from the aluminum cylinder by means of a cork support, stopper and ring assembly. Also, the cylinder has been immersed into an 8-liter stainless steel cooling bath containing alcohol at −25 °C. The cooling bath was put inside an 11 cm thick styrofoam block in order to isolate it from any vibrations and heat transfer to keep the cooling bath temperature at the required temperature during the test very cold for a long period of time. The entire system was covered by a 3 cm thick wooden box. Three T-type thermocouples were used to measure the temperature in the cooling bath; the first one to measure the temperature of the bath, the second one is used to measure Cloud Point, which was placed at the bottom of the glass test jar, while the last one to measured the Pour Point, which was placed in the upper part of the sample in the glass test jar.
Results and Discussion
Accuracy and Repeatability
To ensure the accuracy of the results, an accuracy and repeatability test was carried out. The kinematic viscosity of pure water at 30 °C was found to be 0.803 mm2/s. The value is given as 0.80908 mm2/s [71]. The percent error of the measured value was calculated to be less than 1 %. Table 4 shows the repeatability test results for some samples at specific temperatures. For each sample type, the measurement of the flow time was repeated at each specific temperature four times, and the average flow time was recorded for the calculation of kinematic viscosity. However, with accuracy and repeatability error less than 1 %, it can be concluded that the results to be discussed are 99 % accurate and precise. These measurements were carried out at different temperatures (see Table 4) and atmospheric pressure.
Kinematic Viscosity
From 20 to 270 °C
Figure 4 shows the relationship between the kinematic viscosity and temperature of WFME, WCME, 25-WFME, 50-WFME, and 75-WFME, respectively. It can be seen that viscosity decreases as temperature increases and this behavior can be explained by kinetic molecular theory [72].
Figure 5 shows the change of the kinematic viscosity with the increase of the percentage of WFME to WCME. The x-axis represents the fraction of WCME, whereas the kinematic viscosity values are provided on the y-axis. It can be observed that the kinematic viscosity decreased as the percentage of WFME increased for each temperature considered.
From −10 to 20 °C
Figure 6 and Table 5 provide the kinematic viscosity of biodiesel samples. The tests were initially conducted by 5 °C increments. Toward, the end of the tests 1 °C or 2 °C increments were taken to determine the highest kinematic viscosity of the samples.
It can be seen from Fig. 6 that decreasing the temperature leads to a rapid increase in the kinematic viscosity of biodiesel samples. The formation of crystals of biodiesel samples occurred at various temperatures depending on the percentage of WFME as shown in Table 5.
Density
The density of five samples of biodiesel was measured at a range of temperature, and the results are shown in Fig. 7. It was observed that the density of the blends increased with the percentage of WFME in the blend at all temperatures and decreased with the increase in temperature for all the tested biodiesel samples.
The relationship between the density of the sample and their relative composition percentage at some chosen temperature is shown in Fig. 8.
Dynamic Viscosity
Dynamic viscosity values were calculated from the density and kinematic viscosity by multiplying the measured density and the kinematic viscosity values. The variations in the dynamic viscosity of different blends of WFME and WCME with temperature are shown in Fig. 9. The results showed that, for each of the blends with different percent mixture composition, the viscosity decreased rapidly non-linearly with increasing temperature.
The change of the dynamic viscosity with the increasing of percentage of WFME to WCME at chosen temperatures is shown Fig. 10. The figure shows that the increase of the percentage of WFME leads to a decrease in the dynamic viscosity of the biodiesel.
Cloud Point and Pour Point
Cloud point is the temperature at which small solid crystals are observed at first as the fuel is cooled [73]. In this experiment, the cloud point was definitely the temperature point when the character on the reverse side of the test tube was disappearing due to the formation of crystal wax. Pour Point was determined by cooling down the samples until the biodiesel was totally ceased, and then the degree before the ceasing point was recorded. Pour Points and Cloud Points of biodiesel samples are shown in Table 6. It can be seen from Table 6 and Fig. 11 that increasing the percentage of WFME leads to an increase of the Pour Point and Cloud Point of the biodiesel samples.
Mathematical Models of Biodiesel Samples
A mathematical model is a simple description of physical, chemical or biological processes. Saxena et al. [74] reviewed various methods for the prediction of important thermophysical properties such as cetane number, kinematic viscosity, density, higher heating value, flash point, cloud point, pour point, cold filter plugging point and vapor pressure for various biodiesel feedstocks.
Sivaramakrishnan and Ravikumar [75] developed an equation to calculate cetane number of various vegetable oils and their biodiesel from their viscosity, density, flash point and higher calorific value. They concluded that this equation gives an accuracy of 90 %.
For vegetable oils, it has been shown that density decreases linearly with an increase in temperature. This correlation can be expressed mathematically as in [76],
where \( \rho \). is the density expressed in g cm3, T is the temperature expressed in °C, a is the intercept and b is a negative slope.
According to Krisnangkura et al. [77], viscosity may be considered the integral of the interaction forces of molecules. When heat is applied to fluids, molecules can then slide over each other more easily making the liquid to become less viscous. The effect of temperature on the kinematic viscosity of a given liquid is described by means of the Arrenhius equation
where ν is the kinematic viscosity, mm2/s, Ea the activation energy for flow, R the universal gas constant and T the absolute temperature, K. Additionally, \( A_{1} = \frac{{N_{A} h}}{V} \) where NA, V and h arethe Avogadro’s number, the molecular volume and the Plank’sconstants, respectively. The SI physical unit of kinematic viscosity is m2 s−1. In the case of vegetable oils, Eq. (3) can be rewritten as in Eq. (4), which is known as the Andrade equation [76, 77]
where T is the absolute temperature and A1 and B are specific constants to be adjusted for each specific oil. By applying logarithms to both sides of Eq. (4) we get:
Equation (4) allows us to linearize Eq. (4) by applying the least-squares method and making 1/T the independent variable.Additionally, Azian et al. [78] suggested modifying Eq. (5), which is especially useful when dealing with wide temperature ranges,
Sometimes, the dynamic or absolute viscosity μ is applied, which can be calculated from the kinematic viscosity ν and the density ρ as,
The SI physical unit of dynamic viscosity is the Pa s. The viscosity of biodiesel could be estimated by the empirical equations derived from Grunberg–Nissan’s equation at which the interaction term of the binary pairs of methyl ester has been neglected. These equations were proposed by Ramirez-Verduzco [79].
\( w_{i} \), \( M_{i} \), \( N_{i} \) are the mass fraction (wt%/100), molecular weight in g/mol, and the number of double bond in the fatty acid chain respectively (refer to Table 1). T is the temperature in Kelvin, \( \rho_{b} \) is the density of the biodiesel in g/cm3, \( \mu_{b} \) is the dynam viscosity in mPa s. The kinematic viscosity is estimated from Eqs. (8) and (9).
To predict the viscosities and densities of biodiesel samples at different temperature the correlation between viscosity and temperature and density and temperature, a statistical software package was used to analyze the data. The best correlation between viscosity and temperature as well as between density and temperature for each of biodiesel sample can generally be written as shown in Table 7. Overall, the empirical equations realize the best accuracy for the prediction kinematic viscosity, dynamic viscosity, and density of five samples with the highest correlation coefficient of 0.999. Furthermore, this shows that the empirical equations can be used confidently to predict the biodiesel properties (kinematic, dynamic viscosity and density).
The measurements of the cloud point and pour point were correlated as a function of percentage composition by empirical second order polynomial equations. From the regression coefficient, it was observed that the regression analysis of the data shows that the polynomial equation is better fitted for the measured values than a linear equation [80, 81].
The proposed equations for calculating cloud point and pour point as a function of composition respectively are shown in Table 7.
As both density and viscosity are highly temperature sensitive, it is possible to determine the dependence between them. According to Rodenbush et al. [75], when dealing with vegetable oils, the dependence between density and viscosity can be expressed as illustrated in
Equation (11) is particularly useful because it can be adjusted by means of a least-squares linear regression by choosing 1/ν1/2 as an independent variable.
Table 7 shows the relationship between the density and kinematic viscosity and indicates that equation is accomplished when dealing with the studied biodiesel prepared from waste oil obtained notably high R 2values in all biodiesel samples.
Conclusions and Future Works
The biodiesel samples were prepared via the transesterification method to investigate the biodiesel properties. The kinematic viscosity and density of the prepared biodiesel samples were within the European biodiesel standard and with the exception of the American specifications. In this study, the biodiesel properties (kinematic viscosity, dynamic viscosity and density) and cold flow properties (CP and PP) and kinematic viscosity of the biodiesel samples were measured within the temperature range from 20 to 270 °C and −10 to 20 °C, respectively. The following points have been observed based on the results obtained from the experiments:
-
1.
At high temperatures (above 250 °C), the value of the kinematic viscosity is almost equal and the difference between these values is about 3 %.
-
2.
It has been noticed that the density and the viscosity of the biodiesel sample increase with the increase of the biodiesel fraction. It is also seen that the density and viscosity of each sample decrease with an increase in the temperature.
-
3.
The cloud point and pour point of the WCME increased when the WFME contents of the mixture increase.
-
4.
Empirical equations to predict the density and the viscosity of the biodiesel and its blends as a function of temperature have been developed. The empirical equations and the measured data closely match with R 2 of 0.995 for density, kinematic viscosity, PP and CP model.
-
5.
As a result, it is suggested from the study that it is possible to use the biodiesel obtained from waste oils as an alternative fuel, which can be considered as renewable energy and environmental recycling process from waste oil after frying.
An interesting future study might measure the other properties of the biodiesel samples such as the specific gravity and cold filter plugging point. Moreover, to improve the cold flow properties of the biodiesel samples, some kind of improvers such as conventional diesel fuel (petrodiesel) or alkyl esters can be added.
Since biodiesel can degrade due to oxidation, contact with water, and/or microbial activity, the storage of biodiesel over extended periods may lead to degradation of its fuel properties. Consequently, this research suggests that the degradation process of biodiesel at different temperatures and exposure times should be studied in the future.
References
Bhale, P.V., Deshpande, N.V., Thombre, S.B.: Improving the Low temperature properties of biodiesel fuel. Renew. Energ. 34, 794–800 (2009)
Joshi, R.M., Pegg, M.J.: Flow properties of biodiesel fuel blends at low temperatures. Fuel 86, 143–151 (2007)
Knothe, G.: Biodiesel: current trends and properties. Top. Catal. 53, 714–720 (2010)
American Society for Testing and Materials (ASTM) Standard D 6751. Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. ASTM, Conshohocken
Refaat, A.A.: Different techniques for the production of biodiesel from waste vegetable oil. Int. J. Environ. Sci. Technol. 7(1), 183–213 (2010). doi:10.1007/bf03326130
Yuan, W., Hansen, A.C., Zhang, Q.: Predicting the temperature dependent viscosity of biodiesel fuels. Fuel 88, 1120–1126 (2009)
Jiao, J., Gai, Q.Y., Wei, F.Y., Luo, M., Wang, W., Fu, Y.J., et al.: Biodiesel from forsythia suspense [(Thunb.) vahl (Oleaceae)] seed oil. Bioresour. Technol. 143, 653–656 (2013)
Chu, J., Xu, X., Zhang, Y.: Production and properties of biodiesel produced from Amygdalus pedunculata Pall. Bioresour. Technol. 134, 374–376 (2013)
Knothe, G.: Avocado and olive oil methyl esters. Biomass Bioenergy 58, 143–148 (2013)
Uzun, B.B., Kilic, M., Ozbay, N., Putun, A.E., Putun, E.: Biodiesel production from waste frying oils: optimization of reaction parameters and determination of fuel properties. Energy 44, 347–351 (2012)
Mejía, J.D., Salgado, N., Orrego, C.E.: Effect of blends of diesel and palm-caster biodiesels on viscosity, cloud point and flash point. Ind. Crops Prod. 43, 791–797 (2013)
Knothe, G.: Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 86, 1059–1070 (2005)
Mejía, J.D., Salgado, N., Orrego, C.E.: Effect of blends of diesel and palm-caster biodiesels on viscosity, cloud point and flash point. Ind. Crops Prod. 43, 791–797 (2013)
Knothe, G., Steidley, K.R.: A comparison of used cooking oils: a very heterogeneous feedstock for biodiesel. Bioresour. Technol. 100, 5796–5801 (2009)
Poling, B.E., Prausnitz, J.M., O’Connell, J.P.: The properties of gases and liquids, 5th edn. McGraw-Hill, New York (2001)
Knothe, G., Krahl, J., Van Gerpen, J.H.: The biodiesel handbook, 2nd edn. AOCS Press, Champaign IL (2010)
Hoekman, S.K., Broch, A., Robbins, C., Ceniceros, E., Natarajan, M.: Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 16, 143–169 (2012)
Freitas, S.V.D., Pratas, M.J., Ceriani, R., Lima, A.S., Coutinho, J.A.P.: Evaluation of predictive models for the viscosity of biodiesel. Energ Fuels 25, 352–358 (2011)
Meng, X., Jia, M., Wang, T.: Predicting biodiesel densities over a wide temperature range up to 523 K. Fuel 111, 216–222 (2013)
Esteban, B., Riba, J., Baquero, G., Rius, A., Puig, R.: Temperature dependence of density and viscosity of vegetable oils. Biomass Bioenerg 42, 164–171 (2012)
Moradi, R., Karami, B., Mohadesi, M.: Densities and kinematic viscosities in biodiesel − diesel blends at various temperatures, American chemical society. J. Chem. Eng. Data (2012). doi:10.1021/je3008843
Ustra, M., Silva, F., Ansolin, M., Balen, M., Cantelli, K., Alkimim, I., Mazutti, M., Voll, F., Cabral, V., Cardozo-Filho, L., Corazza, M., Vladimir Oliveira, J.: Effect of temperature and composition on density, viscosity and thermal conductivity of fatty acid methyl esters from soybean, castor and Jatropha curcas oils. J. Chem. Thermodyn. 58, 460–466 (2013)
Verma, P., Sharma, M.P., Dwivedi, G.: Prospects of bio-based alcohols for Karanja biodiesel production: an optimisation study by Response Surface Methodology. Fuel 183, 185–194 (2016)
Bilgin, A., Gülüm, M., Koyuncuoglu, İ., Nac, E., Cakmak, A.: Determination of transesterification reaction parameters giving the lowest viscosity waste cooking oil biodiesel. Procedia Soc. Behav. Sci. 195, 2492–2500 (2015)
Verma, P., Sharma, M.P.: Review of process parameters for biodiesel production from different feedstocks. Renew. Sustain. Energ. Rev. 62, 1063–1071 (2016)
Dwivedi, G., Sharma, M.P.: Application of Box-Behnken design in optimization of biodiesel yield from Pongamia oil and its stability analysis. Fuel 145, 256–262 (2015)
Verma, P., Sharma, M.P.: Comparative analysis of effect of methanol and ethanol on Karanja biodiesel production and its optimisation. Fuel 180, 164–174 (2016)
Bryan, R.: Moser, impact of fatty ester composition on low temperature properties of biodiesel–petroleum diesel blends. Fuel, Elsevier 115, 500–506 (2014)
Mushtaq, M., Tan, I., Nadeem, M., Devi, C., Lee, S., Sagir, M.: A convenient route for the alkoxylation of biodiesel and its influence on cold flow properties. Int. J. Green Energ. 11, 267–279 (2014). doi:10.1080/15435075.2013.772519
Kim, J., Yim, E., Jeon, C., Jung, C., Han, B.: Cold performance of various biodiesel fuel blends at low temperature. Int. J. Automot. Technol. 13(2), 293–300 (2012). doi:10.1007/s12239-012-0027-2
Rasimoglu, N., Temur, H.: Cold flow properties of biodiesel obtained from corn oil. Energy 68, 57–60 (2014)
Nainwal, S., Sharma, N., Sharma, A., Jain, S., Jain, S.: Cold flow properties improvement of Jatropha curcas biodiesel and waste cooking oil biodiesel using winterization and blending. Energy 89, 702–707 (2015)
Verma, P., Sharma, M.P., Dwivedi, G.: Impact of alcohol on biodiesel production and properties. Renew. Sustain. Energ. Rev. 56, 319–333 (2016)
Verma, P., Sharma, M.P.: Performance and emission characteristics of biodiesel fuelled diesel engines. Int. J. Renew. Energ. Res. 5(1), 2015
Verma, P., Sharma, M.P., Dwivedi, G.: Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energ. Reports 2, 8–13 (2016)
Demirbas, A.: Progress and recent trends in biodiesel fuels. Energ. Conserv. Manag. 50, 14–34 (2009)
Bajpai, D., Tyagi, V.K.: Biodiesel: source, production, composition, properties and its benefits. J. Olio Sci. 55, 487–502 (2006)
Ma, F., Hanna, M.A.: Biodiesel production: a review. Bioresour. Technol. 70, 1–15 (1999)
Poonam, N.S., Anoop, S.: Production of liquid biofuels from renewable resources. Prog. Energ. Combust. Sci. 37, 52–68 (2011)
Marchetti, J.M., Miguel, V.U., Errazu, A.F.: Possible methods for biodiesel production. Renew. Sust. Energ. Rev. 11, 1300–1311 (2007)
Helwani, Z., Othman, M.R., Aziz, N., Fernando, W.J.N., Kim, J.: Technologies for production of biodiesel focusing on green catalytic techniques: a review. Fuel Process. Technol. 90, 1502–1514 (2009)
Govinda, R.: Timilsina, and Ashish Shrestha, how much hope should we have for biofuels? Energy (2010). doi:10.1016/j.energy.2010.08.023
Dunn, R.O.: Cold flow properties of biodiesel by automatic and manual analysis methods. J. ASTM Int. 7(4), 1–15 (2010)
Ramos, M.J., Fernández, C.M., Casas, A., Rodríguez, L., Pérez, A.: Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 100, 261–268 (2009)
Giakoumis, E.G.: A statistical investigation of biodiesel physical and chemical properties, and their correlation with the degree of unsaturation. Renew. Energ. 50, 858–878 (2013)
Hoekman, S.K., Broch, A., Robbins, C., Ceniceros, E., Natarajan, M.: Review of biodiesel composition, properties, and specifications. Renew. Sust. Energ. Rev. 16, 143–169 (2012)
Amani, M.A., Davoudi, M.S., Tahvildari, K., Nabavi, S.M., Davoudi, M.S.: Biodiesel production from Phoenix dactylifera as a new feedstock. Ind. Crop Prod. 43, 40–43 (2013)
Moser, B.R.: Influence of blending canola, palm, soybean, and sunflower oil methyl esters on fuel properties of biodiesel. Energ. Fuel 22(6), 4301–4306 (2008)
Sahoo, P.K., Das, L.M.: Process optimization for biodiesel production from Jatropha, Karanja and Polanga oils. Fuel 88, 1588–1594 (2009)
Sanford, S.D., White, J.M., Shah, P.S., Wee, C., Valverde, M.A., Meier, G.R.: Feedstock and biodiesel characteristics report. Renewable Energy Group Inc, Iowa (2010)
Tang, H., Salley, S.O., Simon, K.Y.: Fuel properties and precipitate formation at low temperature in soy, cottonseed, and poultry fat-based biodiesel blends. Fuel 87, 3006–3017 (2008)
Usta, N., Aydoğan, B., Çon, A.H., Uğuzdoğan, E., Özkal, S.G.: Properties and quality verification of biodiesel produced from tobacco seed oil. Energ. Convers. Manag. 52, 2031–2039 (2011)
Al-Shanableh, F., Evcil, A., Savas, M.A.: Prediction of cold flow properties of biodiesel fuel using artificial neural network, Procedia. Comput. Sci. PROCS10116 (in press)
Peel, M.C., Finlayson, B.L., McMahon, T.A.: Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 11, 1633–1644 (2007). doi:10.5194/hess-11-1633-2007
FAO, IIASA, ISRIC, ISSCAS, JRC: Harmonized World Soil Database (version 1.1). FAO, Rome (2009)
ASTM Standard specification for biodiesel fuel (B100) blend stock for distillate fuels. In: Annual Book of ASTM Standards, ASTM International, West Conshohocken, Method D6751-08 (2008a)
Tomes, D.T., Lakshmanan, P., Songstad, D.: Biofuels: Global Impact on Renewable Energy, Production Agriculture, and Technological Advancements. Springer Science + Business Media LLC, New York (2011)
ASTM Standard specification for diesel fuel oil, biodiesel blend (B6 to B20). In: Annual Book of ASTM Standards, ASTM International, West Conshohocken, Method D7467-08a (2008b)
ASTM Standard specification for diesel fuel oils. In: Annual Book of ASTM Standards, ASTM International, West Conshohocken, Method D975-08a (2008c)
Can, O., Celikten, I., Usta, N.: Effects of ethanol addition on performance and emissions of a turbocharged indirect injection Diesel engine running at different injection pressures. Energ. Convers. Manag. 45, 2429–2440 (2004). doi:10.1016/j.enconman.2003.11.024
ASTM Standard specification for fuel oils. In: Annual Book of ASTM Standards, ASTM International, West Conshohocken, Method D396-08b (2008d)
Canakci, M., Van Gerpen, J.: Comparison of engine performance and emissions for petroleum diesel fuel, yellow grease biodiesel, and soybean oil biodiesel. Trans. ASAE 46, 937–944 (2003)
Cennatek Bioanalytical Services.: Kinematic Viscosity According to ASTM D445. http://cennatek.ca/analytical/biodiesel/kinematic-viscosity-according-to-astm-d445/. (2013)
Freitas, Samuel V.D., Pratas, Maria Jorge, Ceriani, Roberta, Lima, Alvaro S., Coutinho, João A.P.: Evaluation of predictive models for the viscosity of biodiesel. Energ. Fuels 2011(25), 352–358 (2010). doi:10.1021/ef101299d
Van Jon, G.: The basics of diesel engines and diesel fuels. In: Knothe, G., Van Gerpen, J., Krahl, J. (eds) The Biodiesel Handbook, 2nd (edn). pp 25–33. ISBN: 978-1-893997-62-2 (2005)
Gerhard, K.: The history of vegetable oil-based diesel fuels. In: Knothe, G., Van Gerpen, J., Krahl, J. (eds). The Biodiesel Handbook, 2nd (edn). pp 12–24. ISBN: 978-1-893997-62-2 (2005a)
Dzida, M., Prusakiewicz, P.: The effect of temperature and pressure on the physicochemical properties of petroleum diesel oil and biodiesel fuel. Fuel 87(10–11), 1941–1948 (2008)
ASTM D446 07: Standard Specifications and Operating Instructions for Glass Capillary Kinematic Viscometers, Philadelphia1966 (1966)
DIN 53012:2003-03: Viscosimetry—capillary viscosimetry of newtonian liquids—sources of errors and corrections, Berlin (2003)
Totten, G.E.: Fuels and lubricants handbook: technology, properties, performance, and testing, Glen Burnie, ISBN: 0-8031-2096-6 (2003)
Pritchard, P.J., Leylegian, J.C.: Introduction to fluid mechanics, eighth edition, united states of america, fox and mcdonald’s, Wiley, New York, ISBN-13 9780470547557
Brown, T.L., LeMay, H.E., Bursten, B.E., Brunauer, L.S.: Chemistry: The Central Science, China (2014)
U.S. Department of Energy: Biodiesel Handling and use Guidelines, DOE/GO-102006-2358, 3rd edition (2006)
Saxena, P., Jawale, S., Joshipura, M.H.A.: Review on prediction of properties of biodiesel and blends of biodiesel. Proc. Eng. 51, 395–402 (2013)
Sivaramakrishnan, K., Ravikumar, P.: Determination of cetane number of biodiesel and its influence on physical properties. ARPN (ARPN) 7, 205–211 (2012)
Rodenbush, C.M., Hsieh, F.H., Viswanath, D.S.: Density and viscosity of vegetable oils. J. Am. Oil Chem. Soc. 76, 1415–1419 (1999)
Krisnangkura, K., Yimsuwan, T., Pairintra, R.: An empirical approach in predicting biodiesel viscosity at various temperatures. Fuel 85, 107–113 (2006)
Azian, M.N., Kamal, A.A.M., Panau, F., Ten, W.K.: Viscosity estimation of triacylglycerols and of some vegetable oils, based on their triacylglycerol composition. J. Am. Oil Chem. Soc. 78, 1001–1005 (2001)
Ramírez-Verduzco, L.F.: Density and viscosity of biodiesel as a function of temperature: empirical models. Renew. Sustain. Energ. Rev. 19, 652–665 (2013)
Joshi, R.M., Pegg, M.J.: Flow properties of biodiesel fuel blends at low temperatures. Fuel 86, 143–151 (2007)
Enweremadu, C.C., Rutto, H.L., Oladeji, J.T.: Full length research paper investigation of the relationship between some basic flow properties of shea butter biodiesel and their blends with diesel fuel. Int. J. Phys. Sci. 6(4), 758–767 (2011)
Acknowledgments
The authors wish to thank the Faculty of Engineering especially the Mechanical Engineering Department at Near East University for their support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kassem, Y., Çamur, H. A Laboratory Study of the Effects of Wide Range Temperature on the Properties of Biodiesel Produced from Various Waste Vegetable Oils. Waste Biomass Valor 8, 1995–2007 (2017). https://doi.org/10.1007/s12649-016-9753-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9753-4