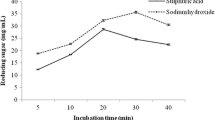
Abstract
The pulverized A. cosmosus peel was found to contain 25 ± 0.31 % cellulose, 28 ± 0.18 % hemicellulose and 8 ± 0.07 % of lignin on dry solid basis. 1 % H2SO4 delignified A. cosmosus peel yielded 38.81 % xylose, 29.31 % fructose and 18.89 % glucose under steam explosion, with a hydrolytic efficiency of 75.52 %. Fourier transform infrared spectroscopy results not only indicated the penetration of H2SO4 in the amorphous region of the biomass and degradation of hemicelluloses but also shows the structural differences before and after pretreatment. Simultaneous Saccharification and Fermentation of pretreated A. cosmosus peel by cellulase and Mucor indicus MTCC 4349 were investigated in the present study. Important process variables for ethanol production from pretreated A. cosmosus peel were optimized using Response Surface Methodology (RSM) based on central composite design (CCD) experiments. A three level CCD experiments with central and axial points was used to develop a statistical model for the optimization of process variables such as incubation temperature (30, 32 and 34 °C) X1, inoculum level (2, 4 and 6 %) X2 and nutrients (1/2/3) X3. Data obtained from RSM on ethanol production were subjected to the analysis of variance and analyzed using a second order polynomial equation and contour plots were used to study the interactions among three relevant variables of the fermentation process. The fermentation experiments were carried out at flask level. The processing parameters setup for reaching a maximum response for ethanol production was obtained when applying the optimum values for temperature (30 °C), inoculum level (2 %) and fermentation medium (urea, NaH2PO4, tryptone and meat extract) for Mucor indicus MTCC 4349. Maximum ethanol concentration 10.4293 g/l was obtained after 72 h from Mucor indicus MTCC 4349 at the optimized process conditions in aerobic batch fermentation.




Similar content being viewed by others
References
Perlack, R.D., Wright, L.L., Turhollow, A.F., Graham, R.L., Sotcks, B.J., Erbach, D.C.: In: Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply. DOE/GO-10 2005-2135, April. US Department of Energy and US Department of Agriculture. Oak Ridge National laboratory. USA, pp. 1–78 (2005)
Wheals, A.E., Basso, L.C., Denise, M., Alves, G., Amorim, H.: Fuel ethanol after 25 years. Trend Biotechnol. 17, 482–487 (1999)
Wang, F.S., Sheu, J.W.: Multiobjective parameter estimation problems of fermentation processes using a high ethanol tolerance yeast. Chem. Eng. Sci. 55, 3685–3695 (2000)
Mitchell, D.: In: A Note of Rising Food Prices. Policy research working paper 4682. Development Prospects Group. pp. 1–21. The World Bank, Washington, DC, USA (2008)
Erukainure, O.L., Ajiboye, J.A., Adejobi, R.O., Okafor, O.Y., Adenekan, S.O.: Protective effect of pineapple (Ananas cosmosus) peel extract on alcohol-induced oxidative stress in brain tissues of male albino rats. Asian Pacific J. Trop. Dis. 1, 5–9 (2011)
Nishio, N., Nagai, S., Leelayuwapan, K.: Ethanol production from pineapple waste. In: Proceedings of conference on energy, Chulalongkorn University, Bangkok (Thailand) (1980) v. 3(pt. 30)
Bhandari, S.V., Panchapakesan, A., Shankar, N., Kumar, H.G.A.: Production of bioethanol from fruit rinds by saccharification and fermentation. Int. J. Sci. Res. Eng. Technol. 2(6), 362–365 (2013)
Smith, B.G., Harris, P.J.: Polysaccharide composition of unlignified cell walls of pineapple [Ananas comosus (L.) Merr.] Fruit. Plant Physiol. 107, 1399–1409 (1995)
Roda, A., De Faveri, D.M., Dordoni, R., Lambri, M.: Vinegar production from pineapple wastes—preliminary saccharification trials. Chem. Eng. Trans. 37 (2014). doi:10.3303/CET1437102
Itelima, J., Onwuliri, F., Onwuliri, E., Onyimba, I., Oforji, S.: Bio-ethanol production from banana, plantain and pineapple peels by simultaneous saccharification and fermentation process. Int. J. Envt. Sci. Dev. 4, 213–216 (2013)
Hansen, M.A.T., Hidayat, B.J., Mogensen, K.K., Jeppesen, M.D., Jørgensen, B., Johansen, K.S., Thygesen, L.G.: Enzyme affinity to cell types in pineapple peel (Triticum aestivum L.) before and after hydrothermal pretreatment. Biotechnol. Biofuels. 6, 54 (2013)
Sharifia, M., Karimi, K., Taherzadeh, M.J.: Production of ethanol by filamentous and yeast like forms of Mucor indicus from fructose, glucose, sucrose and molasses. J. Ind. Microbiol. Biotechnol. 35, 1253–1259 (2008)
Koffi, L.B., Han, Y.W.: Alcohol production from pineapple waste. World J. Microbiol. Biotechnol. 6(3), 281–284 (1990)
Bhatia, L., Johri, S., Ahmad, R.: An economic and ecological perspective of ethanol production from renewable agro-waste- A review. Appl. Microbiol. Biotechnol. Exp. 2, 65 (2012). doi:10.1186/2191-0855-2-65
Tappi Test Methods.: Technical Association of the Pulp and Paper Institute (TAPPI), Atlanta, Georgia, USA (1992)
Szczodrak, J., Fiedurek, J.: Technology for conversion of lignocellulosic biomass to ethanol. Biomass Bioenerg. 10, 367–375 (1996)
Kaar, W.E., Gutierrez, C.V., Kinoshita, C.M.: Steam explosion of sugarcane bagasse as a pretreatment for conversion to ethanol. Biomass Bioenerg. 14(3), 277–287 (1998)
Carvalheiro, F., Duarte, L.C., Lopes, S., Parajo, J.C., Pereira, H., Girio, F.M.: Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Proc. Biochem. 40, 1215–1223 (2005)
Chandel, A.K., da Silva, S.S., Singh, O.V.: Detoxification of Lignocellulose Hydrolysates: biochemical and Metabolic Engineering towards White Biotechnology. Bioenerg Res. 6, 388–401 (2013)
Abdulla, E., Feanov, T., Costa, S., Robra, M.K., Paulo, C.A., Gubitz, M.G.: Decolorization and detoxification of textile dyes with a laccase from Trametes hirsute. Appl. Environ. Microbiol. 66, 3357–3362 (2000)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 (1959)
Singhania, R.R., Skumaran, R.K., Pillai, A., Prema, P., Szakacs, G., Pandey, A.: Solid-state fermentation of lignocellulosic substrates for cellulase production by Trichoderma reesei NRRL 11460. Indian J Biotechnol. 5, 332–336 (2006)
Singh, A., Abidi, A.B., Darmwal, N.S., Agarwal, A.K.: Saccharification of cellulosic substrates by Aspergillus niger cellulase. World J. Microbiol. Biotechnol. 6, 333–336 (1990)
Pasha, C., Valli, N., Rao, L.V.: Lantana camara for fuel ethanol production using thermotolerant yeast. Lett. Appl. Microbiol. 44, 666–672 (2007)
Smidsrod, O., Skjak-Braek, G.: Alginate as an immobilization matrix for cells. Trends Biotechnol. 8(3), 71–78 (1990)
Chandel, A.K., Narasu, M.L., Chandrasekhar, G., Manikyam, A., Rao, L.V.: Use of Saccharum spontaneum (wild sugarcane) as biomaterial for cell immobilization and modulated ethanol production by thermotolerant Saccharomyces cerevisiae VS3. Bioresour. Technol. 100, 2404–2410 (2009)
Giovanni, M.: Response surface methodology and product optimization. J. Food Technol. 37, 41–45 (1983)
Sasikumar, E., Viruthagiri, T.: Simultaneous saccharification and fermentation (SSF) of sugarcane bagasse—kinetics and modeling. Int. J. Chem. Biolog. Eng. 3(2), 57–64 (2010)
Mirahmadi, K., Kabir, M.M., Jeihanipour, A., Karimi, K., Taherzadeh, M.J.: Alkaline pretreatment of spruce and birch to improve bioethanol and biogas production. Bioresources 5(2), 928–938 (2010)
Pandey, K.K., Pitman, A.J.: FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodet. Biodeg. 52, 151–160 (2003)
Oh, S.Y., Yoo, D., Shin, Y., Kim, H.C., Kim, H.Y., Chung, Y.S., Park, W.H., Youk, J.H.: Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohyd. Res. 340, 2376–2391 (2005)
Colom, X., Carrillo, F., Nogues, F., Garriga, P.: Structural analysis of photodegraded wood by means of FTIR spectroscopy. Polym. Degrad. Stab. 80, 543–549 (2003)
Dwivedi, P., Vivekanand, V., Pareek, N., Sharma, A., Singh, R.P.: Bleach enhancement of mixed wood pulp by xylanase-laccase concoction derived through co-culture strategy. Appl. Biochem. Biotechnol. 160, 255–268 (2010)
Pandey, K.K.: A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J. Appl. Polym. Sci. 12, 1969–1975 (1999)
Chandel, A.K., Antunes, F.A., Anjos, V.: Ultra-structural mapping of sugarcane bagasse after oxalic acid fiber expansion (OAFEX) and ethanol production by Candida shehatae and Saccharomyces cerevisiae. Biotechnol. Biofuel. 6, 4 (2013). doi:10.1186/1754-6834-6-4
Berlin, A., Maximenko, V., Gilkes, N., Saddler, J.N.: Optimization of enzyme complexes for lignocellulose hydrolysis. Biotechnol. Bioeng. 97, 287–296 (2007)
Rezende, C.A., de Lima, M.A., Maziero, P., deAzevedo, E.R., Garcia, W., Polikarpov, I.: Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 4, 54 (2011). doi:10.1186/1754-6834-4-54
Tu, M., Chandra, R., Saddler, J.N.: Evaluating the distribution of cellulases and recycling of free cellulases during the hydrolysis of lignocellulosic substrates. Biotechnol. Prog. 23, 398–406 (2007)
Kim, T.H., Taylor, F., Hicks, K.: Bioethanol production from barley hull using SAA (soaking in aqueous ammonia) pretreatment. Biores. Technol. 99, 5694–5702 (2008)
Cheung, S.W., Anderson, B.C.: Laboratory investigation of ethanol production from municipal primary wastewater. Bioresour. Technol. 59, 81–96 (1997)
Huang, X.L., Penner, M.H.: Apparent substrate inhibition of the Trichoderma reesei cellulase system. J. Agric. Food Chem. 39, 2096–2100 (1991)
Penner, M.H., Liaw, E.T.: Kinetic consequences of high ratios of substrate to enzyme saccharification systems based on Trichoderma cellulase. In: Himmel, M.E., Baker, J.O., Overend, R.P. (eds.) Enzymatic Conversion of Biomass for Fuels Production, pp. 363–371. American Chemical Society, Washington, DC (1994)
Saha, B.C., Iten, L.B., Cotta, M.A., Wu, Y.V.: Dilute acid pretreatment, enzymatic saccharification and fermentation of pineapple peel to ethanol. Proc. Biochem. 40, 3693–3700 (2005)
Zheng, Y., Pan, Z., Zhang, R., Wang, D.: Enzymatic saccharification of dilute acid pretreated saline crops for fermentable sugar production. Appl. Ener. 86, 2459–2467 (2009)
Santos, V.T.O., Esteves, P.J., Milagres, A.M.F., Carvalho, W.: Characterization of commercial cellulases and their use in the saccharification of a sugarcane bagasse sample pretreated with dilute sulfuric acid. J. Ind. Microbiol. Biotechnol. 38, 1089–1098 (2011)
Kristensen, J.B., Borjesson, J., Bruun, M.H., Tjerneld, F., Jorgensen, H.: Use of surface active additives in enzymatic hydrolysis of pineapple peel lignocelluloses. Enzyme Microbial. Technol. 40, 888–895 (2007)
Białas, W., Czerniak, A., Szymanowska-Powałowska, D.: Kinetic modeling of simultaneous saccharification and fermentation of corn starch for ethanol production. Acta Biochim. Pol. 61(1), 153–162 (2014)
Białas, W., Szymanowska, D., Grajek, W.: Fuel ethanol production from granular corn starch using Saccharomyces cerevisiae in a long term repeated SSF process with full stillage recycling. Bioresour. Technol. 101, 3126–3131 (2010)
Acknowledgments
We are grateful to Hon’ble Vice Chancellor of Bilaspur University for his constant support. We are grateful to the Department of Zoology, Dr. Hari Singh Gour Central University, Sagar, M.P., India, for help in HPLC analysis work. We are also thankful to Dr Suresh Thareja, Pharmacy department, Guru Ghasidas Central University, Bilaspur, C.G., India, for his help in FTIR analysis work. RSM work was completed in Department of Microbiology and Bioinformatics of Bilaspur University, Bilaspur.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhatia, L., Johri, S. FTIR Analysis and Optimization of Simultaneous Saccharification and Fermentation Parameters for Sustainable Production of Ethanol from Peels of Ananas cosmosus by Mucor indicus MTCC 4349. Waste Biomass Valor 7, 427–438 (2016). https://doi.org/10.1007/s12649-015-9462-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-015-9462-4