Abstract
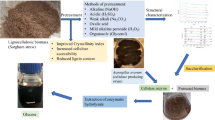
In this study, various pretreatment methods, such as thermal (24 h at 80 °C; 1 h at 120 °C), alkaline (2–20 g ΝaΟΗ/100 g TS, 24 h at 80 °C) and acid (2–20 g Η2SO4/100 g TS, 1 h at 120 οC; 2–20 g Η3PO4/100 g TS, 1 h at 120 °C; 2–20 g HCl/100 g TS, 1 h at 120 °C), were applied on sunflower straw biomass and the effect of each pretreatment method on the fractionation of the lignocellulosic content was evaluated. Experiments showed that pretreatment with the acids led to higher hemicellulose solubilization, while lignin degradation was achieved when the sunflower was treated with NaOH. Higher acids concentration led to higher solubilization of hemicellulose and higher concentrations of released furaldehydes (5-hydroxymethylfurfural and furfural) and aliphatic acids (i.e. formic and acetic acid). On the contrary, the higher the NaOH concentration, the higher was the phenolics concentration observed. Scanning electron microscopy images revealed a change in morphology after different pretreatment methods, compared to the raw substrate. Thus, disruption of the outer layers and removal of mass from the initial connected structure were observed, for all pretreated feedstocks. Complete analysis of the liquid fractions obtained after pretreatment, led to the estimation of the ratio of sum of soluble sugars concentration to the sum of inhibitors concentration (Σ(soluble sugars)/Σ(inhibitors)), which characterizes the fermentability of the sunflower straw hydrolysates.



Similar content being viewed by others
References
European Commission Eurostat: http://epp.eurostat.ec.europa.eu/portal/page/portal/statistics/search database (2010). Accessed 10 Feb 2010
FAOSTAT: http://faostat.fao.org (2013). Accessed 05 Mar 2013
Punda, I.: Investment Centre Division, Sunflower Crude and Refined Oils. Agribusiness Handbook, Italy (2010)
Antonopoulou, G., Alexandropoulou, M., Lyberatos, G.: The effect of thermal, chemical and enzymatic pretreatment on saccharification and methane generation from sunflower straws. In: Proceedings Venice 2010, third international symposium on energy from biomass and waste, Venice, Italy 8–11 November (2010)
Marechal, V., Rigal, L.: Characterization of by-products of sunflower culture- commercial applications for stalks and heads. Ind. Crops Prod. 10, 185–200 (1999)
Monlau, F., Barakat, A., Trably, E., Dumas, C., Steyer, J.-P., Carrere, H.: Lignocellulosic materials into biohydrogen and biomethane: impact of structural features and pretreatment. Crit. Rev. Environ. Sci. Technol. 43, 260–322 (2013)
Saha, B.C., Yoshida, T., Cotta, M.A., Sonomoto, K.: Hydrothermal pretreatment and enzymatic saccharification of corn stover for efficient ethanol production. Ind. Crop. Prod. 44, 367–372 (2013)
Caparros, S., Ariza, J., Lopez, F., Nacimiento, J.A., Garrote, G., Jimenez, L.: Hydrothermal treatment and ethanol pulping of sunflower stalks. Bioresour. Technol. 99, 1368–1372 (2008)
Diaz, M.J., Cara, C., Ruiz, E., Perez-Bonilla, M., Castro, E.: Hydrothermal pretreatment and enzymatic hydrolysis of sunflower stalks. Fuel 90, 3225–3229 (2011)
Ruiz, E., Cara, C., Manzanares, P., Ballesteros, M., Castro, E.: Evaluation of steam explosion pre-treatment for enzymatic hydrolysis of sunflower stalks. Enzyme Microb. Technol. 42, 160–166 (2008)
Cao, S., Pu, Y., Studer, M., Wyman, C., Ragauskas, A.J.: Chemical transformations of populus trichocarpa during dilute acid pretreatment. RSC Adv. 2, 10925–10936 (2012)
Galbe, M., Zacchi, G.: Pretreatment: the key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 46, 70–78 (2012)
Mosier, N., Wyman, C., Dale, B., Elander, R., Lee, Y.Y., Holtzapple, M., Ladisch, M.: Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 96(6), 673–686 (2005)
Chandel, A.K., Singh, O.V., Narasu, M.L., Rao, L.V.: Bioconversion of Saccharum spontaneum (wild sugarcane) hemicellulosic hydrolysate into ethanol by mono and co-cultures of Pichia stipitis NCIM3498 and thermotolerant Saccharomyces cerevisiae-VS3. New Biotechnol. 28(6), 593–599 (2011)
Zheng, Y., Lee, C., Yu, C., Cheng, Y.-S., Zhang, R., Jenkins, B.M., VanderGheynst, J.S.: Dilute acid pretreatment and fermentation of sugar beet pulp to ethanol. Appl. Energy 105, 1–7 (2013)
Martinez, M.L., Sanchez, S., Bravo, V.: Production of xylitol and ethanol by Hansenula polymorpha from hydrolysates of sunflower stalks with phosphoric acid. Ind. Crop Prod. 40, 160–166 (2012)
Marcotullio, G., Krisanti, E., Giuntoli, J., De Jong, W.: Selective production of hemicellulose-derived carbohydrates from wheat straw using dilute HCl or FeCl3 solutions under mild conditions. X-ray and thermo-gravimetric analysis of the solid residues. Bioresour. Technol. 102, 5917–5923 (2011)
Monlau, F., Sambusiti, C., Barakat, A., Quemeneur, M., Trably, E., Steyer, J.-P., Carrère, H.: Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 32, 934–951 (2014)
Ruiz, E., Romero, I., Moya, M., Cara, C., Vidal, J.D., Castro, E.: Dilute sulfuric acid pretreatment of sunflower stalks for sugar production. Bioresour. Technol. 140, 292–298 (2013)
Buranov, A.U., Mazza, G.: Lignin in straw of herbaceous crops. Ind. Crops Prod. 28, 237–259 (2008)
Monlau, F., Aemig, Q., Barakat, A., Steyer, J.-P., Carrère, H.: Application of optimized alkaline pretreatment for enhancing the anaerobic digestion of different sunflower stalks varieties. Environ. Technol. 34(13–14), 2155–2162 (2013)
Monlau, F., Barakat, Α., Steyer, J.-P., Carrère, H.: Comparison of seven types of thermo-chemical pretreatments on the structural features and anaerobic digestion of sunflower stalks. Bioresour. Technol. 120, 241–247 (2012)
Antonopoulou, G., Lyberatos, G.: Effect of pretreatment of sweet sorghum biomass on methane generation. Waste Biomass Valoris. 4, 583–591 (2013)
Larsson, S., Palmqvist, E., Hahn-Hagerdal, B., Tengborg, C., Stenberg, K., Zacchi, G., Nilvebrant, N.-O.: The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 24(3–4), 151–159 (1999)
Panagiotopoulos, I.A., Bakker, R.R., de Vrije, T., Koukios, E.G.: Effect of pretreatment severity on the conversion of barley straw to fermentable substrates and the release of inhibitory compounds. Bioresour. Technol. 102, 11204–11211 (2011)
Lee, J.Y., Ryu, H.J., Oh, K.K.: Acid-catalyzed hydrothermal severity on the fractionation of agricultural residues for xylose-rich hydrolyzates. Bioresour. Technol. 132, 84–90 (2013)
Pedersen, M., Meyer, A.S.: Lignocellulose pretreatment severity—relating pH to biomatrix opening. New Biotechnol. 27(6), 739–750 (2010)
Sluiter, A., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D.: Determination of extractives in biomass. Laboratory Analytical Procedure (LAP), NREL/TP-510-42619. National Renewable Energy Laboratory, Colorado (2008)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D.: Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP), NREL/TP-510-42618. National Renewable Energy Laboratory, Colorado (2008)
Joseffson, B.: Rapid spectrophotometric determination of total carbohydrates. In: Grasshoff, K., Ehrhardt, M., Kremling, K. (eds.) Methods of Seawater Analysis, pp. 340–342. Verlag Chemie GmbH, Berlin (1983)
APHA, AWWA, WPCF.: Standard methods for the examination of water and wastewater. Franson, M.A. (eds.) American Public Health Association, Washington (1995)
Waterman, P.G., Mole, S.: Analysis of phenolic plant metabolites. In: Lawton, J.H., Likens, G.E. (eds.) Methods in Ecology. Blackwell Scientific Publications, Oxford (1994)
Chandel, A.K., da Silva, S.S., Carvalho, W., Singh, O.V.: Sugarcane bagasse and leaves: foreseeable biomass of biofuel and bio-products. J. Chem. Technol. Biotechnol. 87, 11–20 (2012)
Guo, P., Mochidzuki, K., Cheng, W., Zhou, M., Gao, H., Zheng, D., Wang, X., Cui, Z.: Effects of different pretreatment strategies on corn stalk acidogenic fermentation using a microbial consortium. Bioresour. Technol. 102(16), 7526–7531 (2011)
Nizami, A.-S., Korres, N.E., Murphy, J.D.: Review of the integrated process for the production of grass biomethane. Environ. Sci. Technol. 43(22), 8496–8508 (2009)
Monlau, F., Trably, E., Barakat, A., Hamelin, J., Steyer, J.-P., Carrere, H.: Two-stage alkaline–enzymatic pretreatments to enhance biohydrogen production from sunflower stalks. Environ. Sci. Technol. 47, 12591–12599 (2013)
Xie, S., Frost, J.P., Lawlor, P.G., Wu, G., Zhan, X.: Effects of thermo-chemical pre-treatment of grass silage on methane production by anaerobic digestion. Bioresour. Technol. 102(19), 8748–8755 (2011)
Taherzadeh, M.J., Karimi, K.: Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 9, 1621–1651 (2008)
Alemdar, A., Sain, M.: Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 99, 1664–1671 (2008)
Shafiei, M., Karimi, K., Taherzadeh, M.J.: Palm date fibers: analysis and enzymatic hydrolysis. Int. J. Mol. Sci. 11(11), 4285–4296 (2010)
Rasmussen, H., Sorensen, H.R., Meyer, A.S.: Formation of degradation compounds from lignocellulosic biomass in the biorefinery: sugars reaction mechanisms. Carbohydr. Res. 385, 45–57 (2014)
Almeida, J.R.M., Modig, T., Petersson, A., Hahn-Hägerdal, B., Liden, G., Gorwa-Grauslund, M.-F.: Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 82, 340–349 (2007)
Du, B., Sharma, L.N., Becker, C., Chen, S.-F., Mowery, R.A., van Walsum, G.P., Chambliss, C.K.: Effect of varying feedstock-pretreatment chemistry combinations on the formation and accumulation of potentially inhibitory degradation products in biomass hydrolysates. Biotechnol. Bioeng. 107(3), 430–440 (2010)
Almeida, J.R.M., Bertilsson, M., Gorwa-Grauslund, M.F., Gorsich, S., Lidén, G.: Metabolic effects of furaldehydes and impacts on biotechnological processes. Appl. Microbiol. Biotechnol. 82(4), 625–638 (2009)
de Vrije, T., Bakker, R.R., Budde, M.A.W., Lai, M.H., Mars, A.E., Claassen, P.A.M.: Efficient hydrogen production from the lignocellulosic energy crop Miscanthus by the extreme thermophilic bacteria Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Biotechnol. Biofuels 2, 12 (2009)
Taherzadeh, M.J., Eklund, R., Gustafsson, L., Niklasson, C., Liden, G.: Characterization and fermentation of dilute-acid hydrolyzates from wood. Ind. Eng. Chem. Res. 36, 4659–4665 (1997)
Hierholtzer, A., Chatellard, L., Kierans, M., Akunna, J.C., Collier, P.J.: The impact and mode of action of phenolic compounds extracted from brown seaweed on mixed anaerobic microbial cultures. J. Appl. Microbiol. 114(4), 964–973 (2013)
Klinke, H.B., Ahring, B.K., Schmidt, A.S., Thomsen, A.B.: Characterization of degradation products from alkaline wet oxidation of wheat straw. Bioresour. Technol. 82(1), 15–26 (2002)
Quémeneur, M., Hamelin, J., Barakat, A., Steyer, J.P., Carrere, H., Trably, E.: Inhibition of fermentative hydrogen production by lignocellulose-derived compounds in mixed cultures. Int. J. Hydrogen Energy 37(4), 3150–3159 (2012)
Cao, G.-L., Ren, N.-Q., Wang, A.-J., Guo, W.-Q., Xu, J.-F., Liu, B.-F.: Effect of lignocellulose derived inhibitors on growth and hydrogen production by Thermoanaerobacterium thermosaccharolyticum W16. Int. J. Hydrogen Energy 35(24), 13475–13480 (2010)
Ho, K.-L., Chen, Y.-Y., Lee, D.-J.: Biohydrogen production from cellobiose in phenol and cresol-containing medium using Clostridium sp. R1. Int. J. Hydrogen Energy 35, 10239–10244 (2010)
Monlau, F., Aemig, Q., Trably, E., Hamelin, J., Steyer, J.-P., Carrere, H.: Specific inhibition of biohydrogen-producing Clostridium sp. after dilute-acid pretreatment of sunflower stalks. Int. J. Hydrogen Energy 38, 12273–12282 (2013)
Hsu, T.-C., Guo, G.-L., Chen, W.-H., Hwang, W.-S.: Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 101, 4907–4913 (2010)
Naseeruddin, S., Yadav, K.S., Sateesh, L., Manikyam, A., Desai, S., Venkateswar, R.L.: Selection of the best chemical pretreatment for lignocellulosic substrate Prosopis juliflora. Bioresour. Technol. 136, 542–549 (2013)
Acknowledgments
The authors wish to thank the Greek General Secretariat for Research and Technology for the financial support of this work under “Supporting Postdoctoral Researchers Projects”—Pretreatment of lignocellulosic wastes for 2nd generation biofuels (POSTDOC_PE8(1756)) (post-doc fellowship of Dr. G. Antonopoulou).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antonopoulou, G., Dimitrellos, G., Beobide, A.S. et al. Chemical Pretreatment of Sunflower Straw Biomass: The Effect on Chemical Composition and Structural Changes. Waste Biomass Valor 6, 733–746 (2015). https://doi.org/10.1007/s12649-015-9388-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-015-9388-x