Abstract
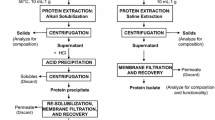
As Jatropha curcas L. is a non-edible energy plant biodiesel production from J. curcas L. crude oil is an important field of interest in order to avoid the ‘tank or table’ discussion. As a by-product from oil extraction, high amounts of Jatropha meals are obtained requiring a concept for its sustainable utilization. Due to the high protein content (up to 40 %) in Jatropha seed cakes, added value can be generated by extraction of these proteins for further applications. The present study compared an aqueous and an enzyme-assisted process for protein extraction from screw-pressed and aqueous de-oiled Jatropha residue. Additionally, different methods for protein recovery were evaluated. Aqueous-extracted proteins were recovered by ultrafiltration, isoelectric precipitation and/or lyophilisation while the proteins from enzyme-assisted extraction were only lyophilized. Functionality of the obtained protein products was determined indicating good emulsifying properties as well as a good gelation behaviour for the aqueous-extracted proteins. In general, proteins from enzyme-assisted extraction had slightly lower functionality. The study indicates potential for the application of Jatropha proteins as emulsifiers in biodegradable bags or as antifoam agents.


Similar content being viewed by others
References
Jongschaap, R.E.E., Corré, W.J., Bindraban, P.S., Brandenburg, W.A.: Claims and Facts on Jatropha curcas L., chapter 1, report no. 158, pp. 1–4. Plant Research International B.V. (2007)
Brittaine, R., Lutaladio, N.: Jatropha: A Smallholder Bioenergy Crop. The Potential for Pro-Poor Development. Integrated Crop Management, chapter 1, vol. 8, pp. 1–12 (2010)
Juan, J.C., Kartika, D.A., Wu, T.Y., Hin, T.Y.Y.: Biodiesel production from jatropha oil by catalytic and non-catalytic approaches: an overview. Bioresou. Technol. 102(2), 452–460 (2011). doi:10.1016/j.biortech.2010.09.093
Nazir, N., Ramli, N., Mangunwidjaja, D., Hambali, E., Setyaningsih, D., Yuliani, S., Yarmo, M.A., Salimon, J.: Extraction, transesterification and process control in biodiesel production from Jatropha curcas. Eur. J. Lipid Sci. Technol. 111(12), 1185–1200 (2009). doi:10.1002/ejlt.200800259
Becker, K., Makkar, H.: Jatropha curcas: a potential source for tomorrow’s oil and biodiesel. Lipid Techno. 20(5), 104–107 (2008)
Makkar, H.P.S., Becker, K., Sporer, F., Wink, M.: Studies on nutritive potential and toxic constituents of different provenances of Jatropha curcas. J. Agric. Food Chem. 45(8), 3152–3157 (1997)
Makkar, H., Becker, K.: Method for detoxifying plant constituents. World Intellectual Property Organization (ed.), WO 2010/092143 A1, Germany, pp. 1–49 (2010)
Wang, X.-H., Ou, L., Fu, L.-L., Zheng, S., Lou, J.-D., Gomes-Laranjo, J., Li, J., Zhang, C.: Detoxification of Jatropha curcas kernel cake by a novel Streptomyces fimicarius strain. J. Hazard. Mater. 260, 238–246 (2013). doi:10.1016/j.jhazmat.2013.05.012
Moure, A., Sineiro, J., Dominguez, H., Parajo, J.C.: Functionality of oilseed protein products: a review. Food Res. Int. 39(9), 945–963 (2006). doi:10.1016/j.foodres.2006.07.002
Alsohaimy, S.A., Sitohy, M.Z., El-Masry, R.A.: Isolation and partial characterization of chickpea, lupine and lentil seed proteins. World J. Agric. Sci. 3(1), 123–129 (2007)
Sussmann, D., Pickardt, C., Schweiggert, U., Eisner, P.: Influence of different processing parameters on the isolation of lupin (Lupinus angustifolius L.) protein isolates: a preliminary study. J. Food Process Eng 36(1), 1–11 (2011)
Lusas, E.W., Riaz, M.N.: Soy protein products—processing and use. J. Nutr. 125(3), S573–S580 (1995)
Vioque, J., Sanchez-Vioque, R., Pedroche, J., Yust, M.D., Millan, F.: Production and uses of protein concentrates and isolates. Grasas Aceites 52(2), 127–131 (2001)
Wasche, A., Muller, K., Knauf, U.: New processing of lupin protein isolates and functional properties. Nahrung 45(6), 393–395 (2001)
Saetae, D., Kleekayai, T., Jayasena, V., Suntornsuk, W.: Functional properties of protein isolate obtained from physic nut (Jatropha curcas L.) seed cake. Food Sci. Biotechnol. 20(1), 29–37 (2011). doi:10.1007/s10068-011-0005-x
Makkar, H.P.S., Francis, G., Becker, K.: Protein concentrate from Jatropha curcas screw-pressed seed cake and toxic and antinutritional factors in protein concentrate. J. Sci. Food Agric. 88(9), 1542–1548 (2008). doi:10.1002/Jsfa.3248
Devappa, R.K., Swamylingappa, B.: Biochemical and nutritional evaluation of Jatropha protein isolate prepared by steam injection heating for reduction of toxic and antinutritional factors. J. Sci. Food Agric. 88(5), 911–919 (2008). doi:10.1002/Jsfa.3170
Sterchi, E.E., Stöcker, W.: Proteolytic enzymes—Tools and targets. Springer, Berlin (1998)
Treimo, J., Aspmo, S.I., Eijsink, V.G.H., Horn, S.J.: Enzymatic solubilization of proteins in brewer’s spent grain. J. Agric. Food Chem. 56(13), 5359–5365 (2008). doi:10.1021/Jf073317s
Apiwatanapiwat, W., Vaithanomsat, P., Somkliang, P., Malapant, T.: Optimization of protein hydrolysate production process from Jatropha curcas cake. Eng. Technol. 53, 109–112 (2009)
Gofferjé, G., Klingele, S., Staebler, A., Schweiggert-Weisz, U.: Comparison of two protein extraction techniques utilizing aqueous de-oiled residue from Jatropha curcas L. Waste Biomass Valoriz. 5, 33–41 (2014). doi:10.1007/s12649-013-9229-8
Ersson, B., Rydén, L., Janson, J.-C.: Introduction to protein purification. In: Janson, J.-C. (ed.) Protein purification—Principles, high resolution methods, and applications, vol. 54, pp. 3–21. Wiley, New Jersey (2011)
Scopes, R.K.: Protein purification—Principles and practise, 3rd edn. Springer Science and Business Media, LLC, New York (1994)
Foster, P.R., Dunnill, P., Lilly, M.D.: Kinetics of protein salting-out—Precipitation of yeast enzymes by ammonium-sulfate. Biotechnol. Bioeng. 18(4), 545–580 (1976). doi:10.1002/bit.260180408
Kozinski, A.A., Lightfoo, E.N.: Protein ultrafiltration—general example of boundary-layer filtration. AIChE J. 18(5), 1030–1040 (1972). doi:10.1002/aic.690180523
Maa, Y.F., Nguyen, P.A., Andya, J.D., Dasovich, N., Sweeney, T.D., Shire, S.J., Hsu, C.C.: Effect of spray drying and subsequent processing conditions on residual moisture content and physical/biochemical stability of protein inhalation powders. Pharm. Res. 15(5), 768–775 (1998). doi:10.1023/A:1011983322594
Masters, K.: Spray drying handbook, 3rd edn. Halsted Press, Sydney (1979)
Makkar, H.P.S., Aderibigbe, A.O., Becker, K.: Comparative evaluation of non-toxic and toxic varieties of Jatropha curcas for chemical composition, digestibility, protein degradability and toxic factors. Food Chem. 62(2), 207–215 (1998). doi:10.1016/S0308-8146(97)00183-0
Hamarneh, A.I., Heeres, H.J., Broekhuis, A.A., Picchioni, F.: Extraction of Jatropha curcas proteins and application in polyketone-based wood adhesives. Int. J. Adhes. Adhes. 30(7), 615–625 (2010). doi:10.1016/j.ijadhadh.2010.06.001
Selje-Assmann, N., Makkar, H.P.S., Hoffmann, E.M., Francis, G., Becker, K.: Quantitative and qualitative analyses of seed storage proteins from toxic and non-toxic varieties of Jatropha curcas L. Eaap Public 124, 625–626 (2007)
Peralta-Flores, L., Gallegos-Tintore, S., Solorza-Feria, J., Davila-Ortiz, G., Chel-Guerrero, L., Martinez-Ayala, A.: Biochemical evaluation of protein fractions from physic nut (Jatropha curcas L.). Grasas Aceites 63(3), 253–259 (2012). doi:10.3989/gya.072511
Usman, L.A., Ameen, O.M., Ibiyemi, S.A., Muhammad, N.O.: The extraction of proteins from the neem seed (Indica azadirachta A-Juss). Afr. J. Biotechnol. 4(10), 1142–1144 (2005)
Lestari, D., Mulder, W.J., Sanders, J.P.M.: Jatropha seed protein functional properties for technical applications. Biochem. Eng. J. 53(3), 297–304 (2011). doi:10.1016/j.bej.2010.12.003
Saetae, D., Suntornsuk, W.: Toxic compound, anti-nutritional factors and functional properties of protein isolated from detoxified Jatropha curcas seed cake. Int. J. Mol. Sci. 12(1), 66–77 (2011). doi:10.3390/Ijms12010066
Kinsella, J.E.: Functional-properties of soy proteins. J. Am. Oil Chem. Soc. 56(3), 242–258 (1979). doi:10.1007/Bf02671468
AOAC: Protein (crude) in animal feed. Official methods of analysis of the Association of Official Analytical Chemists (AOAC) 15 Ed. (1990)
Frister, H., Meisel, H., Schlimme, E.: Opa method modified by use of N, N-dimethyl-2-mercaptoethylammonium chloride as thiol component. Fresen. Z. Anal. Chem. 330(7), 631–633 (1988)
Nielsen, P.M., Petersen, D., Dambmann, C.: Improved method for determining food protein degree of hydrolysis. J. Food Sci. 66(5), 642–646 (2001)
AACC: Method 56-20. Hydration capacity of pregelatinized cereal products. In: Chemists, A.A.o.C. (ed.) Approved Methods of the AACC. AACC International, St. Paul (2000)
Ludwig, I., Ludwig, E., Pingel, B.: Micro-method for the determination of the fat-holding capacity of proteins. Nahrung 33(1), 99–101 (1989)
Coffman, C.W., Garcia, V.V.: Functional properties and amino acid content of protein isolate form mung bean flour. J. Food Technol. 12, 473–484 (1977)
Renkema, J.M.S.: Relations between rheological properties and network structure of soy protein gels. Food Hydrocoll. 18(1), 39–47 (2004). doi:10.1016/S0268-005x(03)00040-7
Sousa, I.M.N., Mitchell, J.R., Ledward, D.A., Hill, S.E., daCosta, M.L.B.: Differential scanning calorimetry of lupin and soy proteins. Z. Lebensm. Unters. For. 201(6), 566–569 (1995)
Morr, C.V., German, B., Kinsella, J.E., Regenstein, J.M., Vanburen, J.P., Kilara, A., Lewis, B.A., Mangino, M.E.: A collaborative study to develop a standardized food protein solubility procedure. J. Food Sci. 50(6), 1715–1718 (1985)
Adler-Nissen, J.: Enzymatic hydrolysis of proteins for increased solubility. J. Agric. Food Chem. 24(6), 1090–1093 (1976)
Rosenthal, A., Pyle, D.L., Niranjan, K.: Simultaneous aqueous extraction of oil and protein from soybean: mechanisms for process design. Food Bioprod. Process. 76(C4), 224–230 (1998). doi:10.1205/096030898532124
de Moura, J.M.L.N., Campbell, K., de Almeida, N.M., Glatz, C.E., Johnson, L.A.: Protein extraction and membrane recovery in enzyme-assisted aqueous extraction processing of soybeans. J. Am. Oil Chem. Soc. 88(6), 877–889 (2011). doi:10.1007/s11746-010-1737-0
Latif, S., Diosady, L.L., Anwar, F.: Enzyme-assisted aqueous extraction of oil and protein from canola (Brassica napus L.) seeds. Eur. J. Lipid Sci. Technol. 110(10), 887–892 (2008). doi:10.1002/ejlt.200700319
Sari, Y.W., Bruins, M.E., Sanders, J.P.M.: Enzyme assisted protein extraction from rapeseed, soybean, and microalgae meals. Ind. Crop Prod. 43, 78–83 (2013). doi:10.1016/j.indcrop.2012.07.014
León-López, L., Dávila-Ortiz, G., Jiménez-Martínez, C., Hernández-Sánchez, H.: Sequentially integrated optimization of the conditions to obtain a high-protein and low-antinutritional factors protein isolate from edible Jatropha curcas seed cake. ISRN Biotechnol. (2013). doi:10.5402/2013/197201
Omosaiye, O., Cheryan, M.: Ultrafiltration of soybean water extracts—processing characteristics and yields. J. Food Sci. 44(4), 1027–1031 (1979). doi:10.1111/j.1365-2621.1979.tb03438.x
Mueller, K., Eisner, P., Yoshie-Stark, Y., Nakada, R., Kirchhoff, E.: Functional properties and chemical composition of fractionated brown and yellow linseed meal (Linum usitatissimum L.). J. Food Eng. 98(4), 453–460 (2010). doi:10.1016/j.jfoodeng.2010.01.028
Kilara, A., Sharkasi, T.Y.: Effects of temperature on food proteins and its implications on functional-properties. Crit. Rev. Food Sci. 23(4), 323–395 (1986)
Raymundo, A., Franco, J., Gallegos, C., Empis, J., Sousa, I.: Effect of thermal denaturation of lupin protein on its emulsifying properties. Nahrung 42(3–4), 220–224 (1998). doi:10.1002/(Sici)1521-3803(199808)42:03/04<220:Aid-Food220>3.0.Co;2-Q
Damodaran, S.: Protein stabilization of emulsions and foams. J. Food Sci. 70(3), R54–R66 (2005)
Dickinson, E.: Flocculation of protein-stabilized oil-in-water emulsions. Colloid Surf. B 81(1), 130–140 (2010). doi:10.1016/j.colsurfb.2010.06.033
Renkema, J.M.S., Gruppen, H., van Vliet, T.: Influence of pH and ionic strength on heat-induced formation and rheological properties of soy protein gels in relation to denaturation and their protein compositions. J. Agric. Food Chem. 50(21), 6064–6071 (2002). doi:10.1021/Jf020061b
Acknowledgments
This study was financially supported by the German Federal Ministry of Education and Research (BMBF). The authors are grateful to GEA Westfalia Separator AG (Germany) for supplying ADJR and to ASA Spezialenzyme GmbH (Germany) for supplying Protease A01 preparation. We thank Mrs. Sigrid Gruppe, Mrs. Evi Müller, Mrs. Elfriede Bischof and Mrs. Sigrid Bergmann for the chemical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gofferjé, G., Zöttl, A., Stäbler, A. et al. Influence of Protein Extraction Techniques of Different De-oiled Residues from Jatropha curcas L. on Protein Recovery and Techno-functional Properties. Waste Biomass Valor 6, 225–235 (2015). https://doi.org/10.1007/s12649-014-9342-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-014-9342-3