Abstract
Using Raman spectroscopy in conjunction with X-ray diffraction (XRD), transmission electron micrographs (TEM-EDP), and scanning electron microscopy (SEM), the local environment of Te atoms and the crystallization behavior of glasses in the system of chemical formula xAg2O. (100 − x)TeO2 (25 ≤ x ≤ 50 mol%) have been examined. Crystalline structure was evident in the XRD spectra of glasses containing 40 and 50 mol% TeO2. On the other hand, an amorphous structure has been observed in glasses with lower Ag2O concentrations (25, 30, and 35 mol%). Thermal heating could be used to crystallize the amorphous glasses’ structure. Using DSC measurements, the treatment's temperature was controlled. The species with good crystallinity include Ag2Te4O9 and Ag2Te2O5. The results of TEM and EDP, as well as both SEM and XRD, revealed that in glasses enriched with Ag2O, crystalline clustered species were formed. Raman data proved that the crystalline clustered is improved as a result of the formation of TeO3 units enriched with nonbridging oxygen bonds. In compositions containing less than 40 mol%, Ag2O plays the role of a glass modifier. At higher Ag2O concentrations, it plays the role of building crystalline clusters of the Ag2Te4O9 and Ag2Te2O5 types.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
1 Introduction
Due to its possible use in optical devices, tellurite glasses have attracted a lot of research [1,2,3,4,5,6,7]. They exhibit high refractive indices and dielectric constants as well as good nonlinear optical transmittance [8,9,10,11,12]. Several structural studies on binary alkali tellurite glasses have been performed [5,6,7,8,9] using a range of structural techniques. Because the tellurium–oxygen polyhedron is so variable, it is difficult to predict how the structural environment distribution changes when the glass composition changes.
The notation Qnm has been proposed for modified telluride glasses [13,14,15,16,17], where n signifies the amount of bridging oxygen (BO), and m determines the coordination number. Because the abundance of each tellurite polyhedron was supposed to be dependent on the species' charge, the number of non-charged tellurite polyhedra (Q44) was thought to decrease as modifier oxide concentration increased. Alternatively, an increase in charged polyhedral (Q34; Q13, Q03) units was simply observed.
The tellurium structural unit with the greatest TeO2 concentration and lowest modifier oxide is a four-coordinated trigonal-bipyramid TeO4 (Q44), which may be generated in the paratellurite (α-TeO2) polymorph of crystalline TeO2. Some of the stretched bonds can simple be broken when the TeO2 concentration was lowered, resulting in a three-coordinated structure known as (Q34; Q13; Q03). A similar approach was considered in alkali-tellurite glass structures [15,16,17]. It was assumed that five tellurium polyhedra can be identified in tellurium glass systems modified by some extent of alkali or silver oxides [13,14,15,16,17].
Using 23Na NMR spectroscopy, the formation of crystalline clusters produced from Na2TeO3 may then be validated [17]. Sodium ions appear to be randomly distributed at low concentrations. At intermediate concentrations, there is also a strong middle-range order. The existence of some crystalline phases in the sodium tellurite system has been confirmed. In sodium-rich glasses, however, Na4Te4O10 [13, 17, 18] and Na2TeO3 may form. Recent studies have shown that Ag2O plays a similar role as played by Na2O in TeO2 glasses since Ag2TeO3 crystalline phases are confirmed to be formed in Ag2O-rich glasses.
The objective of this work is to determine the impact of both glass composition and thermal heat treatment processes on the structure of tellurite glasses. The findings of XRD, TEM-EDP, and Raman spectroscopy of silver telluride glasses and their crystalline derivatives are presented in this study. The transformation of amorphous to crystalline silver tellurite phases gives the material an extra advantage to be used in the biomedical field of application. Additionally ordered tellurite glasses can be used as high refractive indices and dielectric constants materials.
2 Experimental details
2.1 Sample preparation
The melt quenching technique was used to prepare glasses in the system xAg2O. (100 − x)TeO2, (25–50 mol%). Samples are obtained from reagent-grade mixtures of AgNO3 and TeO2 which have been melted in a Pt-Au crucible. To remove NO2, the specimens were heat-treated at a slow rate of 2 °C/min from room temperature to 300 °C and then melted for 20–30 min between 700 and 800 °C before being quenched by pouring the melt between two metallic plates.
2.2 Sample characterization
A Shimadzu X-ray type Dx-30 diffractometer is used for X-ray diffraction measurements. The values of the maximum peak and intensity are used to determine the material type that is compared to patterns in the joint committee for powder diffraction 108 standards’ international powder diffraction file (PDF) database (JCPDS). A transmission electron microscope (TEM) of type JEOL-JEM-1011 was used to determine the size and shape of the studied samples. Microstructural data were tested using the JSM-7500F field emission scanning electron microscope. The machine operated with an accelerated voltage of 25 kV. All samples were sputter-coated with a thin layer of gold (3–4 nm) to avoid sample charging and increase the signal-to-noise ratio. The samples containing 30 and 35 mol% Ag2O were heated in a muffle furnace (Heraeus KR170) with a temperature control of less than 2 °C. The samples were heat-treated at a temperature of 200 °C for a treatment time interval of 10 h. After heating, the glasses were then kept in the furnace and held at the temperature of heat treatment for the desired time before cooling normally at room temperature. Using a Raman confocal microscope, WITec model Alpha 300, with laser power of 532 nm, and 10 mw in the range of 200–2000 cm−1 were recorded. The obtained spectra were deconvoluted to get information about the structural changes of the basic units in these glasses.
3 Results and discussion
3.1 XRD results
Depending on the glass composition, both crystalline and amorphous natures can be possessed in the studied binary silver tellurite glasses. Glasses containing 25, 30, and 35 mol% Ag2O have the amorphous structure which is the most dominant, as reflected from XRD patterns presented in Fig. 1. Higher Ag2O (40 and 50 mol%) concentration leads to the formation of a specific type of crystalline species in the main tellurite glass network.
As shown in Fig. 1, the tendency for crystal formation increases as the Ag2O content increases from 35 to 50 mol%. There are weak diffraction peaks visible in the spectra of glasses containing 35 and 40% mol%. Conversely, glasses with a higher Ag2O content can resolve XRD peaks that are more intense and sharp. The crystalline aggregate of Ag ions in the tellurite network is confirmed to be the source of two distinct diffraction peaks, specifically those at 33° and 38°. This behavior suggests that other silver-containing species may have developed in high silver-containing glasses.
Adding Ag2O at the expense of TeO2 can directly convert TeO4 to other units containing NBO atoms [15,16,17]. High modification levels, on the other hand, can result in the formation of more NBO as well as additional structurally clustered species in the telluride network. At extremely higher Ag2O content (40 and 50 mol%), as in the present study, most Ag2O can be consumed to build crystalline cluster species of type Ag2TeO3 [12,13,14]. This role was identified to be dependent on the bond type formed between Ag cation and oxygen ions. These findings suggest that at high Ag2O concentrations [13, 14], it would be difficult to confirm its modifier role only in the binary-modified glasses, but it can play an additional role that appears in cluster formation.
Based on the above arguments, it can be concluded that TeO4 tetrahedral units are the main forming species in the telluride network. Some ordered TeO4 groups can be converted to TeO3 units after the addition of a modifier [12,13,14,15,16,17,18,19]. Extremely high modifier contents can result in extra TeO3 groups. (The tellurium unit contains one and two nonbridging oxygen atoms.) Furthermore, some crystalline species of type Ag2TeO3 are formed in silver-rich telluride glasses as well as Na2TeO3 crystalline clusters which have been formed in silver and sodium-rich telluride glasses [14, 17, 19].
3.2 TEM-EDP results
Figure 2 presents the TEM micrograph with its electron diffraction pattern (EDP) for two different compassions (30 and 50 mol% Ag2O) that were introduced as examples for the amorphous and crystallized samples, respectively. As shown in Figs. 2(a and b), the main structural groups are distributed separately with an irregular repetition. Furthermore, in the composition of higher Ag2O content (50 mol% Ag2O), the specific types of clusters are seen to be distributed as aggregates or clusters in the host main network. The above arguments agree well with the general concept of cluster formation [8,9,10,11]. In such a situation, the term "cluster" is generally defined as an aggregate or accumulation of bound atoms or molecules that are between the sizes of a molecule and a bulk solid [8, 9]. This argument was supported to a great extent by experimental studies of Ag-rich telluride glasses, which revealed the presence of Ag clusters when the concentration of Ag2O is relatively high (50 mol% or more), see Fig. 2. In the sample of composition 50 mol% Ag2O, the highly crystallized telluride and Ag metallic phases are the dominant phases.
As a general conclusion, all of the investigated glasses of Ag2O/TeO2 ≥ 1 are highly crystallized into homogeneous telluride and Ag metallic phases. But in samples of lower Ag2O/TeO2 molar ratio, on the other hand, a significant difference is considered. The formation of crystalline clusters required more NBO and silver ions to be accumulated.
3.3 SEM–EDS spectra
On a selection of compositions, SEM–EDS analyses were performed to determine how composition affected morphology. Figures 3 and 4 display the SEM images and EDS spectra of glasses with 30 and 50 mol% Ag2O, respectively. There are tellurium, oxygen, and silver peaks to be seen. With an increase in Ag2O content, Ag's silver peak intensity increases. The two peaks for Ag and Te atoms are virtually similar at 30 mol% Ag2O, indicating that Ag2O is only used in TeO2 modification. However, the sample with a 50 mol% Ag content had a higher peak intensity of Ag than Te. The XRD and TEM-EDP results are both confirming the higher level of modification of Ag to form accumulated crystalline clusters. As an alternative, it is also thought that the higher loading of Ag at the expense of Te atoms is the reason for the increase in relative intensity. Then, SEM micrograph of the sample of 30 mol% Ag2O is different from that of the samples of 50 mol%. More overcrowded species were present in the morphology of glass enriched with Ag2O, which could be combined to produce more intense shapes.
3.4 Effect of thermal heating
It can be realized from XRD patterns (Fig. 1) that samples of 25, 30, and 35 mol% Ag2O possessed an amorphous structure. Therefore, the thermal heat treatment at 200 °C provided by the DSC curves (Fig. 5) was shown to have a significant impact on the structure of the presented compositions. The amorphous structure of 25, 30, and 35 mol% Ag2O-containing glasses could be changed into a more regular structure as seen from XRD spectra (Fig. 6) of samples treated thermally at 200 °C for 10 h. The pattern from untreated samples is compared with the XRD patterns in Fig. 6. At 200 °C, the sample is almost completely crystalline, with a little amount of glass phase visible in the background. The ordering of the Ag cations in the primary tellurite network is the polymorph of Ag2Te4O9 [20,21,22]. This crystalline species is thought to be a metastable silver-like compound found in silver telluride samples with compositions of 30 and 35 mol% Ag2O.
3.5 Raman spectra
Raman spectral data are a complementary measurement that may be used for the investigation of the vibrational modes of the molecules that relies upon inelastic scattering [23, 24].
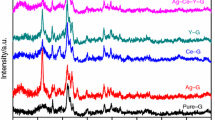
It can be shown from Fig. 7 that both the intensity and relative area of the Raman bands at about (400–500 cm−1) and 600–700 cm−1) assigned to TeO4 and TeO3+1 showed a decreasing trend. Such smearing bands can be assured through the deconvolution analysis technique (DAT) previously discussed by different authors [25,26,27,28,29]. Figure 8 is an example of the deconvoluted spectra for two samples of glasses. The analyzed values of the relative area for each band are listed in Table 1. However, the band representing TeO3 units (between 700 and 800 cm−1) has shown an inverse trend which leads to the formation of NBO in TeO4 units which is transformed into TeO3 units via the formation of NBO atoms. The formation of NBO in the TeO2 network at high Ag2O concentrations (40 and 50 mol%) is considered the main reason for the formation of crystalline clustered species detected by SEM and TEM micrographs.
4 Conclusions
The XRD spectra of glasses in the xAg2O.(100 − x)TeO2 (25 ≤ x ≤ 50 mol%) showed significant changes as the glass composition changed. XRD patterns have shown that some crystalline phases can be formed without thermal treatment. On the other hand, the amorphous glasses containing 25, 30, and 35 mol% Ag2O could be crystallized through a thermal heating process. The Raman spectra have specific features characterizing different telluride structural units. When the glass network becomes rich with Ag2O content, most of the available TeO4 and TeO3+1 units are transformed into TeO3−, TeO32−, and Ag-Te species. The TEM and EDP data agree well with the XRD results, indicating the formation of crystalline clustered species in glasses enriched with Ag2O.
References
C Devaraja, G J Gowda and B Eraiah K. Keshavamurthy Ceram. Inter. 47 7602 (2021)
R El-Mallawany, W M Abou-Taleb, M A Naeem and M E Krar Optik 242 167171 (2021)
N Effendy, H A A Sidek, M K Halimah and M H M Zaid Mater. Chem. Phys. 273 125156 (2021)
A. Okasha, S.Y. Marzouk, A.M. Abdelghany Opt. Laser Technol. 137106829 (2021)
K.I. Hussein, A.M. Al-Syadi, M.S. Alqahtani, N. Elkhoshkhany, H. Algarni, M. Reben, E.S. Yousef Materials 15 2403 (2022)
S B Mallur and P K Babu Mater. Res. Bull. 147 111651 (2022)
M.S. Al-Buriahi, C. Eke, Z.A. Alrowaili, A.M. Al-Baradi, I. Kebaili, B.T. Tonguc Optik 249 168257 (2022)
R G Capelo, J M Almeida, D F Franco, G Y Poirier, C R Mendonça and M Nalin D. Manzani J. Mater. Res. Technol. 13 1296 (2021)
J de Clermont-Gallerande, S Saito, M Colas and P Thomas J. Alloys Compd. 854 157072 (2021)
R.P. Kumbhakar, S.K. Dhiman, S.K. Mahajan, G.F. Ansari Mater. Today: Proc. 56 1313 (2022)
S Suehara, P Thomas, A Mirgorodsky, T Merle-Méjean, J C Champarnaud-Mesjard and T Aizawa J. Non-Cryst. Solids 345 730 (2004)
E M Roginskii, V G Kuznetsov, M B Smirnov, O Noguera, J R Duclère and M Colas J. Phys. Chem. C 121 12365 (2017)
T Sekiya, N Mochida, A Ohtsuka and M Tonokawa Journal of non-crystalline solids 144 128 (1992)
J.C. McLaughlin, S.L. Tagg, J.W. Zwanziger J. Phys. Chem. B 105 67 (2001)
M Soulis, A P Mirgorodsky, T Merle-Méjean, O Masson and P Thomas J. Non-Cryst. Solids 354 143 (2008)
D. Souri, Z.E. Tahan, S.A. Salehizadeh Indian J.Phys. 90 407 (2016)
S Hashim and S K Ghoshal Indian J. Phys. 94 1811 (2020)
H Elkholy, H Othman, I Hager, M Ibrahim and D de Ligny Materials 12 4140 (2019)
P Sharma, D K Kanchan, M Pant and K P Singh Mater. Sci. Appl. 1 59 (2010)
B.V.R. Chowdari, P.P. Kumari Solid State Ion. 86 521 (1996)
D. Souri Indian J.Phys. 89 1277 (2015)
G El-Damrawi, A M Abdelghany, M I Abdelghany and M A Madshal Materialia 16 101095 (2021)
K. Sklepić, M. Vorokhta, P. Mošner, L. Koudelka, A. Mogus-MilankovicJ. Phys. Chem. B 118 12050 (2014)
G El-Damrawi, H M Zahran and A M Abdelghany J. Taibah Univ. Sci. 15 1123 (2021)
A.M. Abdelghany, Silicon, 2 179 (2010)
A.M. Abdelghany & A. Behairy J. Mater. Res. Technol. 9 10491 (2020)
E I Kamitsos and G D Chryssikos J. Mol. Struct. 247 1 (1991)
A.G. Papadopoulos, N.S. Tagiara, E.D. Simandiras & E.I. Kamitsos J. Phys. Chem. B 124 5746 (2020)
N S Tagiara, D Palles, E D Simandiras, V Psycharis, A Kyritsis, E I Kamitsos and J Non-Cryst Solids 457 116 (2017)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
GED suggests the object of study, interpreted the data and wrote early drafts of the manuscript. WAM performed in situ measurements including Raman and XRD spectroscopy. MS and AA conceptualized the experiment and revised the drafts and final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service, or company that could be construed as influencing the position presented in or the review of, the manuscript entitled.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Mohammedi, W., Sherbiny, M., Abdelghany, A.M. et al. Structure and crystallization behavior of binary silver tellurite glasses and glass ceramics. Indian J Phys 97, 2909–2915 (2023). https://doi.org/10.1007/s12648-023-02628-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-023-02628-9