Abstract
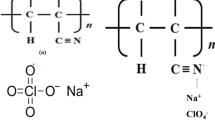
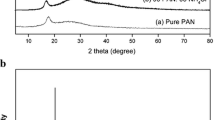
Sodium ion conducting gel polymer electrolytes based on polyacrylonitrile (PAN) with ethylene carbonate and dimethyl formamide as plasticizing solvents are prepared by the solution cast technique. These electrolyte films are free standing, transparent and dimensionally stable. Na+ ions are derived from NaI. The structural properties of pure and complex formations have been examined by X-ray diffraction, Fourier transform infrared spectroscopic studies and differential scanning calorimetric studies. The variation of the conductivity with salt concentration ranging from 10 to 40 wt% is studied. The sample containing 30 wt% of NaI exhibits the highest conductivity of 2.35 × 10−4 S cm−1 at room temperature (303 K) and 1 × 10−3 S cm−1 at 373 K. The conductivity–temperature dependence of polymer electrolyte films obeys Arrhenius behavior with activation energy in the range of 0.25–0.46 eV. The transport numbers both electronic (t e) and ionic (t i) are evaluated using Wagner’s polarization technique. It is revealed that the conducting species are predominantly due to ions. The ionic transport number of highest conducting film is found to be 0.991. Solid-state battery with configuration Na/(PAN + NaI)/(I2 + C + electrolyte) is developed using the highest conducting gel polymer electrolyte system and the discharge characteristics of the cell are evaluated over the load of 100 KΩ.








Similar content being viewed by others
References
R C Agrawal and G P Pandey J. Phys. D Appl. Phys. 41 223001 (2008)
S Sreepathi Rao, M Jaipal Reddy, E Laxmi Narsaiah, U V Subba Rao Mater. Sci. Eng. B 33 173 (1995)
J Y Kim and S H Kim Solid State Ion. 124 91 (1999)
M Jaipal Reddy, T Sreekanth and U V Subba Rao Solid State Ion. 126 55 (1999)
S A Hashmi and S Chandra Mater. Sci. Eng. B34 18 (1995)
A Bhide and K Hariharan Eur. Polym. J. 43 4253 (2007)
V M Mohan, V Raja, A K Sharma and V V R Narasimha Rao Ionics 12 219 (2006)
S Badr, E Sheha, R M Bayomi and M G El-Shaarawy Ionics 16 269 (2010)
G K Prajapati and P N Gupta Physica B 406 3108 (2011)
C V Subba Rao, M Ravi, V Raja, P Balaji Bhargav, A K Sharma and V V R Narasimha Rao Iranian Polym. J. 21 531 (2012)
Kuldeep Mishra, S A Hashmi and D K Rai J. Solid State Electochem. 17 785 (2013)
F M Gray Polymer electrolyte reviews-1 (Newyork: Elsevier) ed. J R MacCallum and C A Vincent 139 (1987)
H Huang, L Chen, X Huang and R Xue Electrochim. Acta 37 1671 (1992)
R Xue, H Huang, M Menetrier and L Chen J. Power Sources 44 431 (1993)
X Huang, L Chen and J Schoonman J. Power Sources 44 487 (1993)
Z Osman, K B Md Isa, A Ahmad, L Othman Ionics 16 431 (2010)
K Vijaya Kumar, G Suneeta Sundari J. Eng. Sci. Tech. 5 130 (2010)
J B Wagner and C Wagner J. Chem. Phys. 26 1597 (1957)
S Rajendran, R Kannan and O Mahendran Mater. Lett. 48 331 (2001)
R M Hodge, G H Edward, G P Simon Polymer 37 1371 (1996)
S K Chaurasia, R K Singh and S Chandra J. Polym. Sci. Part B Polym. Phys. 49 291 (2011)
S K Chaurasia, R K Singh and S Chandra Solid State Ion. 183 32 (2011)
B Huang, Z Wang, L Chen, R Xue, F Wang Solid State Ion. 91 284 (1996)
Z Wang, B Huang, H Huang, R Xue, L Chen, F Wang Spectrochim. Acta Part A 52 691 (1996)
H W Chen, F C Chang J. Polym. Sci. Part B Polym. Phys. 39 2407 (2001)
S K Sidhu, S S Sekhon, S A Hashmi and S Chandra Eur. Polym. J. 29 779 (1993)
E Morales and J L Acosta Solid State Ion. 96 99 (1997)
S Ramesh and A K Arof J. Power Sources 99 41 (2001)
C W Kuo, W B Li, P R Chen, J W Liao, C G Tseng and T YnWu Intern. J. Electrochem. Sci. 8 5007 (2013)
Ch V Subba Reddy, A K Sharma and V V R Narasimha Rao J. Power Sources 111 357 (2002)
W Pan, H Zou Bull. Mater. Sci. 31 807 (2008)
C S Ramya, S Selvasekarapandian, T Savitha, G Hiran Kumar and P C Angelo Phys. B 393 11 (2007)
C S Ramya, T Savitha, S Selvasekharapandian and G Hirankumar Ionics 11 436 (2005)
T Miyamoto and K Shibayana J. Appl. Phys. 44 5372 (1973)
J R Chetia, M Maullick, A Dutta and N N Dass Mater. Sci. Eng. B 107 134 (2004)
K Rama Mohan, V B S Achari, V V R N Rao and A K Sharma Polym. Test. 30 881(2011)
S Sreepathi Rao, M Jaipal reddy, K Narasimha Reddy and U V Subba Rao Solid State Ion. 74 225 (1994)
K Naresh Kumar, T Sreekanth, M Jaipal Reddy and U V Subba Rao J. Power Sources 101 130 (2001)
N S Mohamed, M Z Zakaria, A M M Ali and A K Arof J. Power Sources 66 169 (1997)
A Chandra Indian J. Phys. 87(7) 643 (2013)
A Bhide and K Hariharan Eur. Polym. J. 43 4253 (2007)
P B Bhargav, V M Mohan, A K Sharma and V V R N Rao Ionics 13 173 (2007)
C V Subba Rao, M Ravi, V Raja, P B Bhargav, A K Sharma, V V R N Rao Iranian Polymer J. 21 531 (2012)
Acknowledgments
One of the authors N Krishna Jyothi is very much thankful to Department of Science and Technology (DST), Government of India, New Delhi, for awarding her with a Women Scientist’s scheme under DST-WOS (A) program (File No.: SR/WOS-A/PS-52/2011). The authors thank Er. Koneru Satyanarayana, President and Professor Dr. K. Ravindhranath, Professor in Dept. of Chemistry, for their constant support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishna Jyothi, N., Vijaya Kumar, K., Sunita Sundari, G. et al. Ionic conductivity and battery characteristic studies of a new PAN-based Na+ ion conducting gel polymer electrolyte system. Indian J Phys 90, 289–296 (2016). https://doi.org/10.1007/s12648-015-0758-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-015-0758-9
Keywords
- Gel polymer electrolyte
- Solution casting technique
- DC conductivity
- Transference number
- Discharge characteristics