Abstract
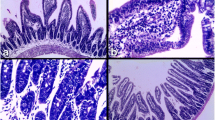
Trichinosis is a sharable parasitic disease caused by Trichinella spp., the disease occurred on eating inappropriate cooked pork infected by the parasite encysted larvae. This study aimed to evaluate experimentally the impact of treatment by thiabendazole, praziquantel (PZQ) and prednisone on T. spiralis induced parasitological, serological and apoptotic changes. Forty albino rats were infected orally each by ± 1000 larvae, divided into four groups each of 10 rats, group (A) infected control, group (B) thiabendazole tested, group (C) PZQ tested and group (D) prednisone tested. On the seventh and 40th days post-infection, all groups were evaluated parasitologically by the number of the intestinal worms and the muscular encysted larvae, while IFN-γ and TNF-α were estimated by ELISA, histopathological and histochemical assessment of the tissue changes during both phases were performed by different stains. In conclusion, thiabendazole was a potent and curable drug, it showed nearly 100% efficacy on intestinal worms, highly significant variations in cytokines levels during both the intestinal and muscular phases, while it induced moderate effects on encysted muscular larvae number, In addition it ameliorated myocytes apoptotic changes induced by trichinosis.



Similar content being viewed by others
References
Andrews P, Thomas H, Ponlke R, Seubert J (1983) Praziquantel. Med Res Rev 3:143–200
Ashour DS, Elbakary RH (2011) Pathogenesis of restricted movements in trichinellosis: an experimental study. Exp Parasitol 128:414–418
Beiting DP, Gagliardo LF, Hesse M, Bliss SK, Meskill D, Appleton JA (2007) Coordinated control of immunity to muscle stage Trichinella spiralis by IL-10, regulatory T cells, and TGF-beta. J Immunol 178:1039–1047
Boonmars T, Wu Z, Nagano I, Takahashi Y (2004) Expression of apoptosis-related factors in muscles infected with Trichinella spiralis. Parasitology 128:323–332
Boonmars T, Wu Z, Nagano I, Takahashi Y (2005) Trichinella pseudospiralis infection is characterized by more continuous and diffuse myopathy than T. spiralis infection. Parasitol Res 97:13–20
Boonmars T, Srirach P, Kaewsamut B, Srisawangwong T, Pinlaor S, Pinlaor P, Yongvanit P, Sithithaworn P (2008) Apoptosis-related gene expression in hamster opisthorchiasis post praziquantel treatment. Parasitol Res 102:447–455
Bratton SB, Walker G, Srinivasula SM, Sun XM, Butterworth M, Alnemri ES, Cohen GM (2001) Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP omplexes. EMBO J 20:998–1009
Brow HD, Matzuk AR, Lives IR, Petrson LH, Harris SA, Sarett LM, Egerton JR, Yakstis JJ, Campbell WC, Cuckler AC (1961) Antiparasitic drugs :IV. (2-4-thia-zolil 1-Benzimidazole) a new anthelmintic. J Am Chem Soc 83:1764–1765
Campbell WC (1994) Meatborne helminth infections: trichinellosis. In: Hui SY, Murrell KD (eds) Food borne disease handbook. Marcel Dekker, New York, pp 255–277
Campbell WC, Cuckler AC (1964) Effect of thiabendazole upon the enteral and parenteral phases of trichinosis in mice. J Parasitol 50:481–486
Carleton MA, Drury GA, Willington EA, Cammeron H, Carleton S (1967) Histological technique, 4th edn. Oxford University Press, New York
Castro DA, Malone C, Smith S (1980) Systemic anti-inflammatory effect associated with enteric trichinosis in the rat. J Parasitol 66:407–412
Chieco P, Derenzini M (1999) The Feulgen reaction 75 years on. Histochem Cell Biol 111:345–358
Dempsey PW, Doyle SE, He JQ, Cheng G (2003) The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev 14:193–209
Denham DA (1965) Studies with methyridine and Trichinella spiralis. I. Effect upon the intestinal phase in mice. Exp Parasitol 17:10–14
Despommier DD (2009) Trichinellida, Dioctophymatida and Enoplean Parasites, Ch. 23. In: Foundations of parasitology, 8th ed. McGraw-Hill companies, Inc., New York, pp 399–418
Dupouy-Camet J, Kociecka W, Bruschi F, Bolas-Fernandez F, Pozio E (2002) Opinion on the diagnosis and treatment of human trichinellosis. Expert Opin Pharmacother 3:1117–1130
Dvorožňáková E, Hurníková Z, Kołodziej-Sobocińska M (2011) Development of cellular immune response of mice to infection with low doses of Trichinella spiralis, Trichinella britovi and Trichinella pseudospiralis larvae. Parasitol Res 108:169–176
El-Ridi AM, Abou-Ragab HA, Ismail MM, Shehata MM, Ramadan ME, Etewa SE (1990) Effect of some drugs on some histopathological and immunological aspects of experimental trichinosis in albino rats. J Egypt Soc Parasitol 20:99–104
Else KJ, Finkelman FD (1998) Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol 28:1145–1158
Fabre MV, Beiting DP, Bliss SK, Appleton JA (2009) Immunity to Trichinella spiralis muscle infection. Vet Parasitol 159:245–248
Frydas S, Papaioannou N, Hatzistilianou M, Merlitti D, Digioaacchino M, Castellanim L, Conti P (2002) Generation of TNFa, IFN-γ, IL-4, IL-6 and IL-10 in Trichinella spiralis infected mice: effect of the anti-inflammatory compound mimosine. Ammo Pharmacology 9(4):389–400
Gamble HR, Bessonov AS, Cuperlovic K, Gajadhar AA, vanKnapen F, Noeckler K, Schenone H, Zhu X (2000) International Commission on trichinellosis: recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet Parasitol 93:393–408
García A, Barrera MG, Piccirilli G, Vasconi MD, Di Masso RJ, Leonardi D, Hinrichsen LI, Lamas MC (2013) Novel albendazole formulations given during the intestinal phase of Trichinella spiralis infection reduce effectively parasitic muscle burden in mice. Parasitol Int 62:568–570
Gottstein B, Pozio E, Nöckler K (2009) Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev 22:127–145
Guenther S, Nöckler K, von Nickisch-Rosenegk M, Landgraf M, Ewers C, Wieler LH, Schierack P (2008) Detection of T. spiralis, T. britovi and T. pseudospiralis in muscle tissue with real-time PCR. J Micro-Boil Methods 75:287–292
Gupta S (2003) Molecular signaling in death receptor and mitochondrial pathways of apoptosis (Review). Int J Oncol 22:15–20
Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE (2000) Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 1:475–482
Helmby H, Grencis RK (2003) IFN-γ independent effects of IL-12 during intestinal nematode infection. J Immunol 171:3691–3696
Hoff J, Rlagt LV (2000) Methods of blood collection in the mouse. Lab Anim 29:47–53
Hosking BC, Watson TG, Leathwick DM (1996) Multigeneric resistance to oxfendazole by nematodes in cattle. Vet Rec 138:67–68
Jarczewdaska K, Gorny M, Zeromski J (1974) Immunological studies of rat skeletal muscles in the course of T. spiralis infection treated with thiabendazole proceedings, 3rd International conference on trichinellosis. Nov. 2–4, 1972. Miami Beach, Florid
Kisiel K, Kaszuba A (2011) Alclometasone dipropionate properties and clinical uses. Post Dermatol Alergol 2:107–119
Kociecka W, Boczon K, Pozio E, van Knapen F (2004) Trichinella. In: Miliotis MD, Bier JW (eds) International handbook of foodborne pathogens. Marcel Dekker, New York, pp 37–658
Lawerence C, Paterson J, Higgins L, MacDonald T, Kennedy M, Garside P (1998) IL-4-regulated enteropathy in an intestinal nematode infection. Eur J Immunol 28:2672–2684
Lee KM, Ko RC (2006) Cell-mediated response at the muscle phase of Trichinella pseudospiralis and Trichinella spiralis infections. Parasitol Res 99:70–77
Leung LM, Barriaga LK (1966) Thiabendazole in the treatment of T. spiralis. Dt Med Wschr 60:70–72
Lundy SK, Lerman SP, Boros DL (2001) Soluble egg antigen simulated T helper lymphocyte apoptosis and evidence for cell death mediated by FasL + T and B cells during murine Schistosoma mansoni infection. Infect Immun 1:271–280
Machnicka B, Katkiewicz M, Dziemian E (1994) Morphopathology of rats infected experimentally with Trichinella spiralis and treated with levamisole or dexamethasone. In: Campbell WC, Pozio E, Bruschi F (eds) Trichinellosis. Instituto Superiore di Sanita Press, Rome, pp 427–432
Matsuo A, Wu Z, Nagano I, Takahashi Y (2000) Five types of nuclei present in the capsule of Trichinella spiralis. Parasitology 121:203–210
Mikkonen TJ, Valkama H, Wihiman S, Sukura A (2005) Spatial variation of Trichinella prevalence in rats in finnish waste disposal sites. J Parasitol 91:210–213
Osborne BA (1996) Apoptosis and the maintenance of homoeostasis in the immune system. Curr Opin Immunol 2:245–254
Piekarska J, Szczypka M, Michalski A, Obmińska-Mrukowicz B, Gorczykowski M (2010) The effect of immunomodulating drugs on the percentage of apoptotic and necrotic lymphocytes in inflammatory infiltrations in the muscle tissue of mice infected with Trichinella spiralis. Pol J Vet Sci 13:233–240
Pozio E (2007) World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol 149:3–21
Pozio E, Rinaldi L, Marucci G, Musella V, Galati F, Cringoli G, Boireau P, La Rosa G (2009) Hosts and habitats of Trichinella spiralis and Trichinella britovi in Europe. Int J Parasitol 39:71–79
Schenone H (1980) Praziquantel in the treatment of Hymenolepis nana infections in children. J Trop Med Hyg 29(320):321
Tato P, Fernández AM, Solano S, Borgonio V, Garrido E, Sepúlveda J, Molinari JL (2004) Acysteine protease from Taenia solium metacestodes induces apoptosis in human CD4+ T-cells. Parasitol Res 92:197–204
Wajant H, Pfizenmaier K, Scheurich P (2003) Tumor necrosis factor signaling. Cell Death Differ 10:45–65
Wu Z, Nagano I, Boonmars T, Takahashi Y (2005a) Tumor necrosis Factor receptor-mediated apoptosis in Trichinella spiralis infected muscle cells. Parasitology 131(Pt 3):373–381
Wu Z, Nagano I, Boonmars T, Takahashi Y (2005b) A spectrum of functional genes mobilized after Trichinella spiralis infection in skeletal muscle. Parasitology 130(5):561–573
Wu Z, Lj Sofronic-Milosavljevic, Nagano I, Takahashi Y (2008) Trichinella spiralis: nurse cell formation with emphasis on analogy to mus-cle cell repair. Parasit Vectors 1:27
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Etewa, S.E., Fathy, G.M., Abdel-Rahman, S.A. et al. The impact of anthelminthic therapeutics on serological and tissues apoptotic changes induced by experimental trichinosis. J Parasit Dis 42, 232–242 (2018). https://doi.org/10.1007/s12639-018-0990-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-018-0990-2