Abstract
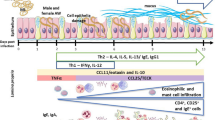
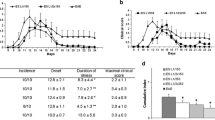
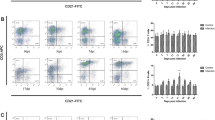
The murine cellular immune response to the infection with ten larvae of encapsulating (Trichinella spiralis, Trichinella britovi) and non-encapsulating species (Trichinella pseudospiralis) was studied. Both T. spiralis and T. britovi stimulated the proliferation of splenic T and B lymphocytes during the intestinal phase of infection, but T. spiralis activated the proliferative response also at the muscle phase, particularly in B cells. Non-encapsulating T. pseudospiralis stimulated the proliferation of T and B cells only on day 10 post-infection (p.i.) and later at the muscle phase. The numbers of splenic CD4 and CD8 T cells of T. spiralis infected mice were significantly increased till day 10 p.i., i.e., at the intestinal phase, and then at the late muscle phase, on day 60 p.i. T. britovi infection increased the CD4 and CD8 T cell numbers only on day 30 p.i. Decreased numbers of CD4 and CD8 T cells after T. pseudospiralis infection suggest a suppression of cellular immunity. Both encapsulating Trichinella species induced the Th2 response (cytokines interleukin-5 (IL-5) and interleukin-10) at the intestinal phase and the Th2 dominant response at the advanced muscle phase. Interferon-γ (IFN-γ) production (Th1 type) started to increase with migrating newborn larvae from day 15 p.i. till the end of the experiment. IL-5 production was suppressed during the intestinal phase of T. pseudospiralis infection. The immune response to T. pseudospiralis was directed more to the Th1 response at the muscle phase, the high IFN-γ production was found on day 10 p.i. and it peaked on days 45 and 60 p.i.







Similar content being viewed by others
References
Andrade MA, Siles-Lucas M, Lopez-Aban J, Nogal-Ruiz JJ, Perez-Arellano JL, Martinez-Fernandez AR, Muro A (2007) Trichinella: differing effects of antigens from encapsulated and non-encapsulated species on in vitro nitric oxide production. Vet Parasitol 143:86–90
Antolová D, Reiterová K, Dubinský P (2006) The role of wild boars (Sus scrofa) in circulation of trichinellosis, toxocarosis and ascariosis in the Slovak Republic. Helminthologia 43:92–97
Beiting DP, Bliss SK, Schlafer DH, Roberts VL, Appleton JA (2004) Interleukin-10 limits local and body cavity inflammation during infection with muscle-stage Trichinella spiralis. Infect Immun 72:3129–3137
Bolas-Fernández F (2003) Biological variation in Trichinella species and genotypes. J Helminthol 77:111–118
Boles LH, Montgomery JM, Morris J, Mann MA, Stewart GL (2000) Suppression of multiple sclerosis in the rat during infection with Trichinella pseudospiralis. J Parasitol 86:841–844
Boonmars T, Wu Z, Nagano I, Takahashi Y (2005) Trichinella pseudospiralis infection is characterized by more continuous and diffuse myopathy than T. spiralis infection. Parasitol Res 97:13–20
Cui J, Wang ZQ, Han HM (2006) Congenital transmission of Trichinella spiralis in experimentally infected mice. Helminthologia 43:7–10
Despommier DD (1998) How does Trichinella spiralis make itself at home? Parasitol Today 14:318–323
Dvorožňáková E, Porubcová J, Šnábel V, Fedoročko P (2008) Immunomodulative effect of liposomized muramyltripeptide phosphatidylethanolamine (L-MTP-PE) on mice with alveolar echinococcosis and treated with albendazole. Parasitol Res 103:919–929
Dvorožňáková E, Hurníková Z, Kołodziej-Sobocińska M (2010) Kinetics of specific humoral immune response of mice infected with low doses of Trichinella spiralis, T. britovi, and T. pseudospiralis larvae. Helminthologia 47:152–157
Dzik JM, Golos B, Jagielska E, Kapala A, Walajtys-Rode E (2002) Early response of guinea-pig lungs to Trichinella spiralis infection. Parasite Immunol 24:369–379
Finkelman FD, Sheadonohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF (1997) Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Ann Rev Immunol 15:505–533
Frydas S, Karagouni E, Dotsika E, Reale M, Barbacane RC, Vlemmas I, Anogianakis G, Trakatellis A, Conti P (1996) Generation of TNF alpha, IFN gamma, IL-6, IL-4 and IL-10 in mouse serum from trichinellosis: effect of the anti-inflammatory compound 4-deoxypyridoxine (4-DPD). Immunol Lett 49:179–184
Furze RC, Selkirk ME (2005) Comparative dynamics and phenotype of the murine immune response to Trichinella spiralis and Trichinella pseudospiralis. Parasite Immunol 27:181–188
Helmby H, Grencis RK (2003) Contrasting roles for IL-10 in protective immunity to different life cycle stages of intestinal nematode parasites. Eur J Immunol 33:2382–2390
Horie S, Gleich GJ, Kita H (1996) Cytokines directly induce degranulation and superoxide production from human eosinophils. J Allergy Clin Immunol 98:371–381
Ishikawa N, Wakelin D, Mahida YR (1997) Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology 113:542–549
Kapel CMO, Gamble HR (2000) Infectivity, persistence, and antibody response to domestic and sylvatic Trichinella spp. in experimentally infected pigs. Int J Parasitol 30:215–221
Khan WI, Vallance BA, Blennerhassett PA, Deng Y, Verdu EF, Matthaei KI, Collins SM (2001) Critical role for signal transducer and activator of transcription factor 6 in mediating intestinal muscle hypercontractility and worm expulsion in Trichinella spiralis-infected mice. Infect Immun 69:838–844
Lee KM, Ko RC (2006) Cell-mediated response at the muscle phase of Trichinella spiralis infections. Parasitol Res 99:70–77
Li CKF, Ko RC (2001) Inflammatory response during the muscle phase of Trichinella spiralis and T. pseudospiralis infections. Parasitol Res 87:708–714
Mahida YR (2003) Host-parasite interactions in rodent nematode infections. J Helminthol 77:125–131
Malakauskas A, Kapel CM, Webster P (2001) Infectivity, persistence and serological response of nine Trichinella genotypes in rats. Parasite 8:S216–S222
Milcheva R, Petkova S, Babál P (2009) Detection of O-glycosylated proteins from different Trichinella species muscle larvae total extracts. Helminthologia 46:139–144
Miterpáková M, Hurníková Z, Antolová D, Dubinský P (2009) Endoparasites of red fox (Vulpes vulpes) in the Slovak Republic with the emphasis on zoonotic species Echinococcus multilocularis and Trichinella spp. Helminthologia 46:73–79
Morales MA, Mele R, Sanchez M, Sacchini D, De Giacomo M, Pozio E (2002) Increased CD8(+)-T-cell expression and a type 2 cytokine pattern during the muscular phase of Trichinella infection in humans. Infect Immun 70:233–239
Morgan UM (2000) Detection and characterisation of parasites causing emerging zoonoses. Int J Parasitol 30:1407–1421
Mosmann TR (1994) Properties and functions of interleukin-10. Adv Immunol 56:1–26
Murrell KD, Bruschi F (1994) Clinical trichinellosis. Prog Clin Parasitol 4:117–150
Paraličová Z, Kinčeková J, Schréter I, Jarčuška P, Dubinský P Jr, Porubčin Š, Pavlinová J, Kristian P (2009) Outbreak of trichinellosis in eastern Slovakia. Helminthologia 46:209–213
Reiterová K, Antolová D, Hurníková Z (2009) Humoral immune response of mice infected with low doses of Trichinella spiralis muscle larvae. Vet Parasitol 159:232–235
Robinson MW, Grieg R, Beattie KA, Lamont DJ, Connolly B (2007) Comparative analysis of the excretory-secretory proteome of the muscle larva of Trichinella pseudospiralis and Trichinella spiralis. Int J Parasitol 37:139–148
Ros-Moreno RM, de Armas-Serra C, Gimenez-Pardo C, Rodriguez-Caabeiro F (2002) Comparison of cholinestase activities in the excretion-secretion products of Trichinella pseudospiralis and Trichinella spiralis muscle larvae. Parasite 9:153–159
Sacchi L, Corona S, Gadjadhar AA, Pozio E (2001) Ultrastructural characteristics of nurse cell-larva complex of four species of Trichinella in several hosts. Parasite 8:S54–S58
Šoltýs J, Quinn MT (1999) Modulation of endotoxin- and enterotoxin-induced cytokine release by in vivo treatment with beta-(1, 6)-branched beta-(1, 3)-glucan. Infect Immun 67:244–252
Stewart GL, Wood B, Boley RB (1985) Modulation of host response by Trichinella pseudospiralis. Parasite Immunol 7:223–233
Turčeková Ľ, Borošková Z, Tomašovičová O, Reiterová K, Kinčeková J (1997) Immunochemical analysis of larval antigens of Trichinella spiralis and T. pseudospiralis. Helminthologia 34:241–243
Vallance BA, Galeazzi F, Collins SM, Snider DP (1999) CD4 T cells and major histocompatibility complex class II expression influence worm expulsion and increased intestinal muscle contraction during Trichinella spiralis infection. Infect Immun 67:6090–6097
Wakelin D, Farias SE, Bradley JE (2002) Variation and immunity to intestinal worms. Parasitology 125:S39–S50
Wang CH (1997) Study of biological properties of Trichinella spiralis newborn larvae and the antiparasitic mucosal immunity of the host. Front Biosci 2:317–330
Wu Z, Matsuo A, Nakada T, Nagano I, Takahashi Y (2001) Different response of satellite cells in the kinetics of myogenic regulatory factors and ultrastructural pathology after Trichinella spiralis and T. pseudospiralis infection. Parasitology 123:85–94
Acknowledgements
This study was supported by the Slovak VEGA agency, grant no. 2/0071/08. The experimental protocols complied with the current Slovak ethic law.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dvorožňáková, E., Hurníková, Z. & Kołodziej-Sobocińska, M. Development of cellular immune response of mice to infection with low doses of Trichinella spiralis, Trichinella britovi and Trichinella pseudospiralis larvae. Parasitol Res 108, 169–176 (2011). https://doi.org/10.1007/s00436-010-2049-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2049-x