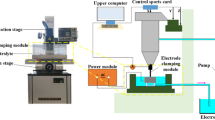
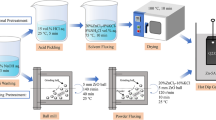
Abstract
The colloidal silica is used as the abrasive for the copper Chemical Mechanical Polishing slurry in integrated circuit multilayer copper wiring. The aggregation of colloidal silica in the slurries tends to aggregate spontaneously, resulting in the continuous changes of the polishing effect, such as scratch defects, removal rate, etc. This situation can lead to the potential instability of the polishing slurry, which should be avoided in industrial production. In this paper, the aggregation and dispersion properties of polishing slurries were systematically studied, and an scheme was proposed to improve the dispersion of slurries to prevent agglomeration. These affecting factors including slurries’ pH, Potassium nitrate and Sodium Polyacrylate were assessed with Large particle counts, Zeta potential and Particle size. Slurry’s pH at 9.6 had a lower Zeta potential which meant a higher dispersion colloidal silica compared to other pH of slurries. And the dissolution of colloidal silica was verified by UV-vis experiment. Sodium dodecyl sulfate, Dodecyl trimethyl ammonium chloride and Primary Alcobol Ethoxylate-15 were analyzed for their dispersion in slurries from the point of view of static electricity and steric hindrance, and the synergistic mechanism of mixed Sodium dodecyl sulfate and Primary Alcobol Ethoxylate-15 on slurries dispersion was highlighted. In addition, the effect of the improvement of slurry dispersion on reducing scratching defects on the surface of polished copper blanket was also tested in this paper.
Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article.
References
Krishnan M, Nalaskowski JW, Cook LM (2010) Chemical mechanical planarization: slurry chemistry, materials, and mechanisms. Chem Rev 110:178–204
Ghulghazaryan R, Wilson J, Abouzeid A (2015) FEOL CMP modeling: Progress and challenges. 2015 International Conference on Planarization/CMP Technology (ICPT), pp 1-4
Wang W, Zhang B, Shi Y, Ma T, Zhou J, Wang R, Wang H, Zeng N (2021) Improvement in chemical mechanical polishing of 4H-SiC wafer by activating persulfate through the synergistic effect of UV and TiO2. J Mater Process Technol 295:117150
Li Y, Liu Y, Wang C, Niu X, Ma T, Xu Y (2018) Role of dispersant agent on scratch reduction during copper barrier chemical mechanical planarization. ECS J Solid State Sci Technol 7:P317–P322
Khanna AJ, Gupta S, Kumar P, Chang F-C, Singh RK (2018) Study of agglomeration behavior of chemical mechanical polishing slurry under controlled shear environments. ECS J Solid State Sci Technol 7:P238–P242
Chang F-C, Kumar P, Singh R, Balasundaram K, Lee J, Lee J, Singh RK (2011) Role of interparticle forces during stress-induced agglomeration of CMP slurries. Colloids Surf A 389:33–37
Basim GB, Moudgil BM (2002) Effect of soft agglomerates on CMP slurry performance. J Colloid Interface Sci 256:137–142
Basim GB, Vakarelski IU, Moudgil BM (2003) Role of interaction forces in controlling the stability and polishing performance of CMP slurries. J Colloid Interface Sci 263:506–515
Remsen EE, Anjur S, Boldridge D, Kamiti M, Li S, Johns T, Dowell C, Kasthurirangan J, Feeney P (2006) Analysis of large particle count in fumed silica slurries and its correlation with scratch defects generated by CMP. J Electrochem Soc 153:G453
Kim Y-H, Kim S-K, Park J-G, Paik U (2010) Increase in the adsorption density of anionic molecules on ceria for defect-free STI CMP. J Electrochem Soc 157:H72
Lei H, Tong K (2016) Preparation of La-doped colloidal SiO2 composite abrasives and their chemical mechanical polishing behavior on sapphire substrates. Precis Eng 44:124–130
Choi W, Mahajan U, Lee S-M, Abiade J, Singh RK (2004) Effect of slurry ionic salts at dielectric silica CMP. J Electrochem Soc 151:G185
Liu G, Liu Y, Niu X, Zhang W, Wang C, Yang S, Ma T (2018) Effects of large particles on MRR, WIWNU and surface quality in TEOS chemical mechanical polishing based on FA/O alkaline slurry. ECS J Solid State Sci Technol 7:P624–P633
Allen LH, Matijević E (1969) Stability of colloidal silica: I. Effect of simple electrolytes. J Colloid Interface Sci 31:287–296
Depasse J, Watillon A (1970) The stability of amorphous colloidal silica. J Colloid Interface Sci 33:430–438
Borisov KM, Bokova ES, Kalinina AA, Svidchenko EA, Bystrova AV, Sumina AM, Moeller M, Muzafarov AM (2020) Formation of hollow silica spheres from molecular colloidal silicas. Mendeleev Commun 30:809–811
Mahadevan TS, Garofalini SH (2008) Dissociative chemisorption of water onto silica surfaces and formation of hydronium ions. J Phys Chem C 112:1507–1515
Braun K, Pochert A, Beck M, Fiedler R, Gruber J, Lindén M (2016) Dissolution kinetics of mesoporous silica nanoparticles in different simulated body fluids. J Sol-Gel Sci Technol 79:319–327
Xiao Y, Lasaga AC (1994) Ab initio quantum mechanical studies of the kinetics and mechanisms of silicate dissolution: H+(H3O+) catalysis. Geochim Cosmochim Acta 58:5379–5400
Mazer JJ, Walther JV (1994) Dissolution kinetics of silica glass as a function of pH between 40 and 85°C. J Non-Cryst Solids 170:32–45
Hua Z, Zhang J, Bai X, Ye Z, Tang Z, Liang L, Liu Y (2016) Aggregation of TiO2–graphene nanocomposites in aqueous environment: Influence of environmental factors and UV irradiation. Sci Total Environ 539:196–205
Dong S, Cai W, Xia J, Sheng L, Wang W, Liu H (2021) Aggregation kinetics of fragmental PET nanoplastics in aqueous environment: Complex roles of electrolytes, pH and humic acid. Environ Pollut 268:115828
Grasso D, Subramaniam K, Butkus M, Strevett K, Bergendahl J (2002) A review of non-DLVO interactions in environmental colloidal systems. Rev Environ Sci Biotechnol 1:17–38
Márquez-Beltrán C, Langevin D (2007) Electrostatic effects in films stabilised by non-ionic surfactants. J Colloid Interface Sci 312:47–51
Ambrose RJ (1989) Surfactants and interfacial phenomena—2nd edn, by Milton J, Rosen, Wiley, 431. J Polym Sci Part C: Polym Lett 27:503-503
Ho NFH, Toguchi H, Higuchi WI (1973) Comparison of theoretical equations for potential energy of electrostatic repulsion of colloidal particles at constant surface charge. J Pharm Sci 62:851–853
Mori T, Kitamura K (2017) Effect of adsorption behaviour of polyelectrolytes on fluidity and packing ability of aqueous graphite slurries. Adv Powder Technol 28:280–287
Chin CH, Muchtar A, Azhari CH, Razali M, Aboras M (2015) Optimization of pH and dispersant amount of Y-TZP suspension for colloidal stability. Ceram Int 41:9939–9946
Li C-C, Liu W-I, Chen Y-S(2017) Efficient dispersants for the dispersion of gallium zinc oxide nanopowder in aqueous suspensions. J Am Ceram Soc 100:920–928
Kim H-M, Prasanna Venkatesh R, Kwon T-Y, Park J-G(2012) Influence of anionic polyelectrolyte addition on ceria dispersion behavior for quartz chemical mechanical polishing. Colloids Surf A 411:122–128
Wei Q, Meng Y, He J, Tang G (2010)Chemical-mechanical dispersing behavior of a nanoceria abrasive. J Rare Earths 28:478–481
Chen J, He T, Wu W, Cao D, Yun J, Tan CK (2004) Adsorption of sodium salt of poly(acrylic) acid (PAANa) on nano-sized CaCO3 and dispersion of nano-sized CaCO3 in water. Colloids Surf A 232:163–168
Hang J, Shi L, Feng X, Xiao L (2009) Electrostatic and electrosteric stabilization of aqueous suspensions of barite nanoparticles. Powder Technol 192:166–170
Elhaei R, Kharrat R, Madani M (2021) Stability, flocculation, and rheological behavior of silica suspension-augmented polyacrylamide and the possibility to improve polymer flooding functionality. J Mol Liq 322:114572
Matusiak J, Grządka E (2020) Cationic starch as the effective flocculant of silica in the presence of different surfactants. Sep Purif Technol 234:116132
Maurya NK, Mandal A (2018) Investigation of synergistic effect of nanoparticle and surfactant in macro emulsion based EOR application in oil reservoirs. Chem Eng Res Des 132:370–384
Al-Anssari S, Arif M, Wang S, Barifcani A, Iglauer S (2017) Stabilising nanofluids in saline environments. J Colloid Interface Sci 508:222–229
Shao P, Pei J, Tang H, Yu S, Yang L, Shi H, Yu K, Zhang K, Luo X (2021)Defect-rich porous carbon with anti-interference capability for adsorption of bisphenol A via long-range hydrophobic interaction synergized with short-range dispersion force. J Hazard Mater 403:123705
Palla BJ, Shah DO (2002) Stabilization of high ionic strength slurries using surfactant mixtures: molecular factors that determine optimal stability. J Colloid Interface Sci 256:143–152
Ntakirutimana S (2019) Enhanced surface activity of activated carbon by surfactants synergism. RSC Adv 9:26519-26531-22019 v.26519 no.26545
Khan R, Inam MA, Khan S, Jiménez AN, Park DR, Yeom IT. The Influence of Ionic and Nonionic Surfactants on the Colloidal Stability and Removal of CuO Nanoparticles from Water by Chemical Coagulation. International Journal of Environmental Research and Public Health. 2019; 16(7):1260.
Palla BJ, Shah DO (2000) Stabilization of high ionic strength slurries using the synergistic effects of a mixed surfactant system. J Colloid Interface Sci 223:102–111
Luo C, Xu Y, Zeng N, Ma T, Wang C, Liu Y (2020) Synergy between dodecylbenzenesulfonic acid and isomeric alcohol polyoxyethylene ether for nano-scale scratch reduction in copper chemical mechanical polishing. Tribol Int 152:106576
Kwon T-Y, Ramachandran M, Park J-G(2013) Scratch formation and its mechanism in chemical mechanical planarization (CMP). Friction 1:279–305
Saka N, Eusner T, Chun JH (2010) Scratching by pad asperities in chemical–mechanical polishing. CIRP Ann 59:329–332
Huo FW, Jin ZJ, Zhang R (2010) Study on the effect of nonionic surfactant in copper CMP slurry. Adv Mater Res 135:30–35
Seo Y, Park J, Elaiyaraju P (2014) Effects of pump-induced particle agglomeration during chemical mechanical planarization (CMP). In: Proceedings of International Conference on Planarization/CMP Technology, pp 254-258
Han X, Gan YX (2013) Investigation the complex dynamic evolvement mechanism of particle cluster and surface integrity in the chemical mechanical planarization. Int J Adv Manuf Technol 64:13–22
Funding
This paper was supported by the Major National Science and Technology Special Projects (No.2016ZX02301003-004-007).
Author information
Authors and Affiliations
Contributions
Nengyuan Zeng: Conceptualization, Writing - original draft.
Hongdong Zhao: Resources, Writing - review & editing.
Yuling Liu: Resources, Supervision, Project administration, Funding acquisition.
Chenwei Wang: Formal analysis, Validation.
Wantang Wang: Investigation.
Tengda Ma: Investigation.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Research Involving Human Participants and/or Animals
Not applicable.
Informed Consent
Informed consent was obtained from all authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, N., Zhao, H., Liu, Y. et al. Optimizing of the Colloidal Dispersity of Silica Nanoparticle Slurries for Chemical Mechanical Polishing. Silicon 14, 7473–7481 (2022). https://doi.org/10.1007/s12633-021-01448-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-021-01448-y