Abstract
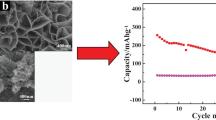
Natural minerals-based energy materials have attracted enormous attention because of the advantages of good materials consistency, high production, environmental friendliness, and low cost. The uniform distribution of grains can effectively inhibit the aggregation of active materials, improving lithium storage performance. In this work, natural graphite is modified by polyvinylpyrrolidone to obtain modified graphite with reduced size and better dispersion. Natural pyrite composite polyvinylpyrrolidone-modified graphite (pyrite/PG) material with uniform particle distribution is obtained by the ball milling process. The subsequent calcination process converts pyrite/PG into Fe1−xS compounded with polyvinylpyrrolidone-modified graphite (Fe1−xS/PG). The homogeneous grain distributions of active material can facilitate the faster transfer of electrons and promote the efficient utilization of active materials. The as-prepared Fe1−xS/PG electrode exhibits a remarkably reversible specific capacity of 613.0 mAh·g−1 at 0.2 A·g−1 after 80 cycles and an excellent rate capability of 523.0 mAh·g−1 at 5 A·g−1. Even at a higher current density of 10 A·g−1, it can deliver a specific capacity of 348.0 mAh·g−1. Moreover, the dominant pseudocapacitance in redox reactions accounts for the impressive rate and cycling stability. This work provides a low-cost and facile method to fabricate natural mineral-based anode materials and apprise readers about the impact of uniform particle distribution on lithium storage performance.
Similar content being viewed by others
References
R.J. He, G.L. Tian, S.P. Li, et al., Enhancing the reversibility of lithium cobalt oxide phase transition in thick electrode via low tortuosity design, Nano Lett., 22(2022), No. 6, p. 2429.
G. Li, T. Ouyang, T.Z. Xiong, et al., All-carbon-frameworks enabled thick electrode with exceptional high-areal-capacity for Li-Ion storage, Carbon, 174(2021), p. 1.
J. Li, D. Yan, S. Hou, et al., Metal-organic frameworks derived yolk-shell ZnO/NiO microspheres as high-performance anode materials for lithium-ion batteries, Chem. Eng. J., 335(2018), p. 579.
H. Zhao, Y. Bai, H. Jin, J. Zhou, X. Wang, and C. Wu, Unveiling thermal decomposition kinetics of Single-Crystalline Ni-Rich LiNi0.88Co0.07Mn0.05O2 cathode for safe Lithium-Ion batteries, Chem. Eng. J., 435(2022), art. No. 134927.
J. Yuan, J.W. Zhu, R.H. Wang, et al., 3D few-layered MoS2/graphene hybrid aerogels on carbon fiber papers: A freestanding electrode for high-performance lithium/sodium-ion batteries, Chem. Eng. J., 398(2020), art. No. 125592.
X.Z. Liu, Y.H. Wang, Y.J. Yang, et al., A MoS2/Carbon hybrid anode for high-performance Li-ion batteries at low temperature, Nano Energy, 70(2020), art. No. 104550.
J.B. Li, J.L. Li, Z.B. Ding, et al., In-situ encapsulation of Ni3S2 nanoparticles into N-doped interconnected carbon networks for efficient lithium storage, Chem. Eng. J., 378(2019), art. No. 122108.
H. Yang, T.Z. Xiong, Z.X. Zhu, et al., Deciphering the lithium storage chemistry in flexible carbon fiber-based self-supportive electrodes, Carbon Energy, 4(2022), No. 5, p. 820.
Y.C. Huang, H. Yang, T.Z. Xiong, et al., Adsorption energy engineering of nickel oxide hybrid nanosheets for high areal capacity flexible lithium-ion batteries, Energy Storage Mater., 25(2020), p. 41.
C.D. Wang, M.H. Lan, Y. Zhang, et al., Fe1−xS/C nanocomposites from sugarcane waste-derived microporous carbon for high-performance lithium ion batteries, Green Chem., 18(2016), No. 10, p. 3029.
M. Shao, Y.Y. Cheng, T. Zhang, et al., Designing MOFs-derived FeS2@Carbon composites for high-rate sodium ion storage with capacitive contributions, ACS Appl. Mater. Interfaces, 10(2018), No. 39, p. 33097.
N. Cheng, X. Chen, L. Zhang, and Z. Liu, Reduced graphene oxide doping flower-like Fe7S8 nanosheets for high performance potassium ion storage, J. Energy Chem., 54(2021), p. 604.
A.H. Jin, M.J. Kim, K.S. Lee, S.H. Yu, and Y.E. Sung, Spindle-like Fe7S8/N-doped carbon nanohybrids for high-performance sodium ion battery anodes, Nano Res., 12(2019), No. 3, p. 695.
S.Z. Huang, Y. Li, S. Chen, et al., Regulating the breathing of mesoporous Fe0.95S1.05 nanorods for fast and durable sodium storage, Energy Storage Mater., 32(2020), p. 151.
P. Jing, Q. Wang, B. Wang, X. Gao, Y. Zhang, and H. Wu, Encapsulating yolk-shell FeS2@carbon microboxes into interconnected graphene framework for ultrafast lithium/sodium storage, Carbon, 159(2020), p. 366.
Q. Wang, C. Tang, D.G. Sun, et al., Coupling Fe3O4/Fe1−xS@Carbon with carbon-coated MoS2 nanosheets as a superior anode for sodium-ion batteries, Chem. Eng. J., 427(2022), art. No. 131652.
L.Y. Zhang, Y.S. Zhang, Y.L. Han, et al., Bead-milling and recrystallization from natural marmatite to Fe-doping ZnS−C materials for lithium-ion battery anodes, Electrochim. Acta, 399(2021), art. No. 139430.
W.Q. Zhao, L.M. Zhang, F. Jiang, et al., Engineering metal sulfides with hierarchical interfaces for advanced sodium-ion storage systems, J. Mater. Chem. A, 8(2020), No. 10, p. 5284.
F. Jiang, Y.C. Bai, L.M. Zhang, et al., Modified bornite materials with high electrochemical performance for sodium and lithium storage, Energy Storage Mater., 40(2021), p. 150.
S.B. Son, T.A. Yersak, D.M. Piper, et al., A stabilized PAN-FeS2 cathode with an EC/DEC liquid electrolyte, Adv. Energy Mater., 4(2014), No. 3, art. No. 1300961.
P. Ge, L.M. Zhang, W.Q. Zhao, Y. Yang, W. Sun, and X.B. Ji, Interfacial bonding of metal-sulfides with double carbon for improving reversibility of advanced alkali-ion batteries, Adv. Funct. Mater., 30(2020), No. 16, art. No. 1910599.
F. Jiang, L.M. Zhang, W.Q. Zhao, et al., Microstructured sulfur-doped carbon-coated Fe7S8 composite for high-performance lithium and sodium storage, ACS Sustainable Chem. Eng., 8(2020), No. 31, p. 11783.
Y.E. Xiang, L.Q. Xu, L. Yang, et al., Natural stibnite for lithium-/sodium-ion batteries: Carbon dots evoked high initial coulombic efficiency, Nanomicro Lett., 14(2022), No. 1, art. No. 136.
J.R. He, Q. Li, Y.F. Chen, et al., Self-assembled cauliflower-like FeS2 anchored into graphene foam as free-standing anode for high-performance lithium-ion batteries, Carbon, 114(2017), p. 111.
D. Li, M.B. Müller, S. Gilje, R.B. Kaner, and G.G. Wallace, Processable aqueous dispersions of graphene nanosheets, Nat. Nanotechnol., 3(2008), No. 2, p. 101.
A.S. Wajid, S. Das, F. Irin, et al., Polymer-stabilized graphene dispersions at high concentrations in organic solvents for composite production, Carbon, 50(2012), No. 2, p. 526.
J.X. Lin, Y.J. Huang, S. Wang, and G.H. Chen, Microwave-assisted rapid exfoliation of graphite into graphene by using ammonium bicarbonate as the intercalation agent, Ind. Eng. Chem. Res., 56(2017), No. 33, p. 9341.
M. Vanitha, P. Camellia, and N. Balasubramanian, Augmentation of graphite purity from mineral resources and enhancing % graphitization using microwave irradiation: XRD and Raman studies, Diam. Relat. Mater., 88(2018), p. 129.
F. Tuinstra and J.L. Koenig, Raman spectrum of graphite, J. Chem. Phys., 53(1970), No. 3, p. 1126.
H. Wu, W.F. Zhao, H.W. Hu, and G.H. Chen, One-step in situ ball milling synthesis of polymer-functionalized graphene nanocomposites, J. Mater. Chem., 21(2011), No. 24, p. 8626.
A. El Din Mahmoud, A. Stolle, and M. Stelter, Sustainable synthesis of high-surface-area graphite oxide via dry ball milling, ACS Sustainable Chem. Eng., 6(2018), No. 5, p. 6358.
A.C. Ferrari, J.C. Meyer, V. Scardaci, et al., Raman spectrum of graphene and graphene layers, Phys. Rev. Lett., 97(2006), No. 18, art. No. 187401.
S.K. Bhargava, A. Garg, and N.D. Subasinghe, In situ high-temperature phase transformation studies on pyrite, Fuel, 88(2009), No. 6, p. 988.
W.H. Chen, X.X. Zhang, L.W. Mi, et al., High-performance flexible freestanding anode with hierarchical 3D carbon-networks/Fe7S8/graphene for applicable sodium-ion batteries, Adv. Mater., 31(2019), No. 8, art. No. 1806664.
J.H. Lu, F. Lian, L.L. Guan, Y.X. Zhang, and F. Ding, Adapting FeS2 micron particles as an electrode material for lithium-ion batteries via simultaneous construction of CNT internal networks and external cages, J. Mater. Chem. A, 7(2019), No. 3, p. 991.
K.H. Ye, Y. Li, H. Yang, et al., An ultrathin carbon layer activated CeO2 heterojunction nanorods for photocatalytic degradation of organic pollutants, Appl. Catal. B, 259(2019), art. No. 118085.
Y.X. Wang, D.M. Chen, J.N. Zhang, et al., Charge relays via dual carbon-actions on nanostructured BiVO4 for high performance photoelectrochemical water splitting, Adv. Funct. Mater., 32(2022), No. 13, art. No. 2112738.
H.H. Fan, H.H. Li, K.C. Huang, et al., Metastable marcasite-FeS2 as a new anode material for lithium ion batteries: CNFs-improved lithiation/delithiation reversibility and Li-storage properties, ACS Appl. Mater. Interfaces, 9(2017), No. 12, p. 10708.
Z. Li, W. Wang, M.J. Zhou, et al., In-situ self-templated preparation of porous core–shell Fe1−xS@N,S co-doped carbon architecture for highly efficient oxygen reduction reaction, J. Energy Chem., 54(2021), p. 310.
Y. Zhang, J. Li, Z. Gong, J. Xie, T. Lu, and L. Pan, Nitrogen and sulfur co-doped vanadium carbide MXene for highly reversible lithium-ion storage, J. Colloid Interface Sci., 587(2021), p. 489.
J.H. Lv, J.T. Du, H.N. Jia, et al., Hierarchical carbon-coated Fe1-xS/mesocarbon microbeads composite as high-performance lithium-ion batteries anode, Ceram. Int., 46(2020), No. 7, p. 9485.
X.X. Xu, Q.N. Ma, Z.H. Zhang, et al., Pomegranate-like mesoporous microspheres assembled by N-doped carbon coated Fe1-xS nanocrystals for high-performance lithium storage, J. Alloys Compd., 797(2019), p. 952.
C.Z. Zhang, D.H. Wei, F. Wang, et al., Highly active Fe7S8 encapsulated in N-doped hollow carbon nanofibers for high-rate sodium-ion batteries, J. Energy Chem., 53(2021), p. 26.
X. Li, T. Liu, Y.X. Wang, et al., S/N-doped carbon nanofibers affording Fe7S8 particles with superior sodium storage, J. Power Sources, 451(2020), art. No. 227790.
S. Zhang, J. Mi, H. Zhao, W. Ma, L. Dang, and L. Yue, Electrospun N-doped carbon nanofibers confined Fe1−xS composite as superior anode material for sodium-ion battery, J. Alloys Compd., 842(2020), art. No. 155642.
Y. Zhang, G.G. Zhao, X. Lv, et al., Exploration and size engineering from natural chalcopyrite to high-performance electrode materials for lithium-ion batteries, ACS Appl. Mater. Interfaces, 11(2019), No. 6, p. 6154.
H.C. Jin, S. Xin, C.H. Chuang, et al., Black phosphorus composites with engineered interfaces for high-rate high-capacity lithium storage, Science, 370(2020), No. 6513, p. 192.
W.J. Yu, C. Liu, L.L. Zhang, et al., Synthesis and electrochemical lithium storage behavior of carbon nanotubes filled with iron sulfide nanoparticles, Adv. Sci., 3(2016), No. 10, art. No. 1600113.
A.K. Haridas, J. Heo, Y. Liu, et al., Boosting high energy density lithium-ion storage via the rational design of an FeS-incorporated sulfurized polyacrylonitrile fiber hybrid cathode, ACS Appl. Mater. Interfaces, 11(2019), No. 33, p. 29924.
Y.Y. Yao, J.C. Zheng, Z.Y. Gong, et al., Metal-organic framework derived flower-like FeS/C composite as an anode material in lithium-ion and sodium-ion batteries, J. Alloys Compd., 790(2019), p. 288.
Y. Xiao, J.Y. Hwang, and Y.K. Sun, Micro-intertexture carbon-free iron sulfides as advanced high tap density anodes for rechargeable batteries, ACS Appl. Mater. Interfaces, 9(2017), No. 45, p. 39416.
F.J. Zhao, L.Y. Yang, Z. Wang, et al., Enhancing lithium storage performance of metal sulfide compound via Fe1−xS/SnS@C complementary heterostructure design, J. Power Sources, 536(2022), art. No. 231460.
Y. Wu, Y.Y. Wang, S.Q. Shao, et al., Transformation of two-dimensional iron sulfide nanosheets from FeS2 to FeS as high-rate anodes for pseudocapacitive sodium storage, ACS Appl. Energy Mater., 3(2020), No. 12, p. 12672.
Q.Q. Xiong, X.J. Teng, J.J. Lou, et al., Design of pyrite/carbon nanospheres as high-capacity cathode for lithium-ion batteries, J. Energy Chem., 40(2020), p. 1.
Y. Wang, X.M. Guo, Z.K. Wang, et al., Controlled pyrolysis of MIL-88A to Fe2O3@C nanocomposites with varied morphologies and phases for advanced lithium storage, J. Mater. Chem. A, 5(2017), No. 48, p. 25562.
S. Shi, M. Zhang, T. Deng, T. Wang and G. Yang, A facile strategy to construct binder-free flexible carbonate composite anode at low temperature with high performances for lithiumion batteries, Electrochim. Acta, 246(2017), p. 1004.
W. Chen, Q.Z. Li, H.X. Zhang, X.H. Wu, W.W. Wu, and M. Xu, Rational synthesis of fern leaf-like FeS2@Sulfur-doped carbon as an anode for superior lithium-ion batteries, Energy Fuels, 35(2021), No. 15, p. 12599.
S. Yuan, W. Zhao, Z. Zeng, et al., Engineering hierarchical Sb2S3/N−C from natural minerals with stable phase-change towards all-climate energy storage, J. Mater. Chem. A, 10(2022), No. 10, p. 5488.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51974222 and 52034011). The authors would like to thank Shiyanjia Lab (www.shiyanjia.com) for the TEM, XPS, and XRD analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
The authors declare no conflicts of interest.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yu, J., Wei, Y., Meng, B. et al. Homogeneous distributed natural pyrite-derived composite induced by modified graphite as high-performance lithium-ion batteries anode. Int J Miner Metall Mater 30, 1353–1362 (2023). https://doi.org/10.1007/s12613-023-2598-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-023-2598-5