Abstract
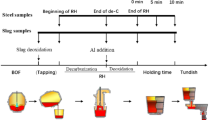
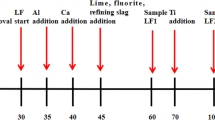
As a key step in secondary refining, the deoxidation process in clean stainless steel production is widely researched by many scholars. In this study, vacuum oxygen decarburization (VOD) deoxidation refining in a 40-t electric arc furnace + VOD + ingot casting process was analyzed and optimized on the basis of Al deoxidation of stainless steel and thermodynamic equilibrium reactions between the slag and steel. Under good stirring conditions in VOD, the deoxidation reaction reaches equilibrium rapidly, and the oxygen activity in the bulk steel is controlled by the slag composition and Al content. A basicity of 3–5 and an Al content greater than 0.015wt% in the melt resulted in an oxygen content less than 0.0006wt%. In addition, the dissolved oxygen content decreased slightly when the Al content in the steel was greater than 0.02wt%. Because of the equilibrium of the Si–O reaction between the slag and steel, the activity of SiO2 will increase while the Si content increases; thus, the Si content should be lowered to enable the formation of a high-basicity slag. A high-basicity, low-Al2O3 slag and an increased Si content will reduce the Al consumption caused by SiO2 reduction.
Similar content being viewed by others
References
D.H. Mesa, A. Toro, A. Sinatora, and A.P. Tschiptschin, The effect of testing temperature on corrosion-erosion resistance of martensitic stainless steels, Wear, 255(2003), No. 1-6, p. 139.
Z.Y. Yang, Z.B. Liu, J.X. Liang, Y.Q. Sun, and W.H. Li, Development of maraging stainless steel, Trans. Mater. Heat Treat., 29(2008), No. 4, p. 1.
H. Ohta and H. Suito, Thermodynamics of aluminum and manganese deoxidation equilibria in Fe-Ni and Fe-Cr alloys, ISIJ Int., 43(2003), No. 9, p. 1301.
H. Todoroki and K. Mizuno, Effect of silica in slag on inclusion compositions in 304 stainless steel, deoxidized with aluminum, ISIJ Int., 44(2004), No. 8, p. 1350.
W.Y. Cha, D.S. Kim, Y.D. Lee, and J.J. Pak, A thermody namic study on the inclusion formation in ferritic stainless steel melt, ISIJ Int., 44(2004), No. 7, p. 1134.
J.H. Park, Thermodynamic investigation on the formation of inclusions containing MgAl2O4 spinel during 16Cr-14Ni austenitic stainless steel manufacturing processes, Mater. Sci. Eng. A, 472(2008), No. 1-2, p. 43.
J.W. Kim, S.K. Kim, D.S. Kim, Y.D. Lee, and P.K. Yang, Formation mechanism of Ca-Si-Al-Mg-Ti-O inclusions in type 304 stainless steel, ISIJ Int., 36(1996), Suppl., p. S140.
Y.H. Sun, Y.N. Zeng, R. Xu, and K.K. Cai, Formation mechanism and control of MgO-Al2O3 inclusions in non-oriented silicon steel, Int. J. Miner. Metall. Mater., 21(2014), No. 11, p. 1068.
Z.Y. Deng and M.Y. Zhu, Evolution mechanism of non-metallic inclusions in Al-killed alloyed steel during secondary refining process, ISIJ Int., 53(2013), No. 3, p. 450.
K. Suzuki, S. Ban-Ya, and M. Hino, Deoxidation equilibrium of Cr-Ni stainless steel with Si at the temperatures from 1823 to 1923 K, ISIJ Int., 42(2002), No. 2, p. 146.
S.B. Lee, J.H. Choi, H.G. Lee, P.C. Rhee, and S.M. Jung, Aluminum deoxidation equilibrium in liquid Fe-16 pct Cr alloy, Metall. Mater. Trans. B, 36(2005), No. 3, p. 414.
H. Suito and R. Inoue, Thermodynamics on control of inclusions composition in ultra-clean steels, ISIJ Int., 36(1996), No. 5, p. 528.
S. Nurmi, S. Louhenkilpi, and L. Holappa, Optimization of intensified silicon deoxidation, Steel Res. Int., 84(2013), No. 4, p. 323.
H. Ohta and H. Suito, Activities in CaO-SiO2-Al2O3 slags and deoxidation equilibria of Si and Al, Metall. Mater. Trans. B, 27(1996), No. 6, p. 943.
X.H. Wang, M. Jiang, B. Chen, and H.B. Li, Study on formation of non-metallic inclusions with lower melting temperatures in extra low oxygen special steels, Sci. China Technol. Sci., 55(2012), No. 7, p. 1863.
J. Zhang, F.M. Wang, and C.R. Li, Thermodynamic analysis of the compositional control of inclusions in cutting-wire steel, Int. J. Miner. Metall. Mater., 21(2014), No. 7, p. 647.
FactSage: http://wwwfactsagecom.
G.K. Sigworth and J.F. Elliott, The thermodynamics of liquid dilute iron alloys, Met. Sci., 8(1974), No. 1, p. 298.
G.P. Wang, Z.B. Li, and C.L. Liu, Effect of VOD & LF processes on stainless steel cleanliness, [in] The 7th CSM Steel Congress, Beijing, 2009, p. 1262.
D. Guo and G.A. Irons, Modeling of gas-liquid reactions in ladle metallurgy: Part I. Physical modeling, Metall. Mater. Trans. B, 31(2000), No. 6, p. 1447.
K. Yamaguchi, Y. Kishimoto, T. Sakuraya, T. Fujii, M. Aratani, and H. Nishikawa, Effect of refining conditions for ultra low carbon steel on decarburization reaction in RH degasser., ISIJ Int., 32(1992), No. 1, p. 126.
J.D. Seo, S.H. Kim, and K.R. Lee, Thermodynamic assessment of the Al deoxidation reaction in liquid iron, Steel Res. Int, 69(1998), No. 2, p. 49.
H. Itoh, M. Hino, and S. Ban-Ya, Assessment of Al deoxidation equilibrium in liquid iron, Tetsu-to-Hagane, 83(1997), No. 12, p. 773.
Z.Y. Deng and M.Y. Zhu, Deoxidation mechanism of Al-killed steel during industrial refining process, ISIJ Int., 54(2014), No. 7, p. 1498.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Lc., Bao, Yp., Wang, M. et al. Variation and optimization of acid-dissolved aluminum content in stainless steel. Int J Miner Metall Mater 23, 408–416 (2016). https://doi.org/10.1007/s12613-016-1250-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-016-1250-z