Abstract
Objective
Dual-energy X-ray absorptiometry (DXA)-derived appendicular lean soft tissue mass (aLM) is used to diagnose sarcopenia. However, DXA-derived aLM includes non-skeletal muscle components, such as fat-free component of adipose tissue fat cell. These components, if not accounted for, could falsely inflate the aLM in individuals with a high amount of adipose tissue mass. B-mode ultrasound accurately measures muscle size in older adults. We sought to develop regression-based prediction equations for estimating DXA-rectified appendicular lean tissue mass (i.e. DXA-derived aLM minus appendicular fat-free adipose tissue (aFFAT); abbreviated as aLM minus aFFAT) using B-mode ultrasound.
Design
Cross-sectional study.
Measurements
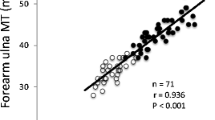
Three hundred and eighty-nine Japanese older adults (aged 60 to 79 years) volunteered in the study. aLM was measured using a DXA, and muscle thickness (MT) was measured using ultrasound at nine sites. An ordinary least-squares multiple linear regression model was used to predict aLM minus aFFAT from sex, age and varying muscle thicknesses multiplied by height. Based on previous studies, we chose to use 4 MT sites at the upper and lower extremities (4-site MT model) and a single site (1-site MT model) at the upper extremity to develop prediction models.
Results
The linear prediction models (4 site MT model; R2 = 0.902, adjusted R2 = 0.899, and 1-site MT model; R2 = 0.868, adjusted R2 = 0.866) were found to be stable and accurate for estimating aLM minus aFFAT. Bootstrapping (n=1000) resulted in optimism values of 0.0062 (4-site MT model) and 0.0036 (1-site MT model).
Conclusion
The results indicated that ultrasound MT combined with height, age and sex can be used to accurately estimate aLM minus aFFAT in older Japanese adults. Newly developed ultrasound prediction equations to estimate aLM minus aFFAT may be a valuable tool in population-based studies to assess age-related rectified lean tissue mass loss.







Similar content being viewed by others

References
Carmeli E. Frailty and Primary Sarcopenia: A Review. Adv Exp Med Biol 2017;1020:53–58
Rizzoli R, Reginster JY, Arnal JF et al. Quality of life in sarcopenia and frailty. Calcif Tissue Int 2013;93:101–120
Hairi NN, Cumming RG, Naganathan V et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc 2010;58: 2055–2062
Lauretani F, Russo CR, Bandinelli S et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003;95: 1851–1860
McLean RR, Shardell MD, Alley DE et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The Foundation for the National Institutes of Health (FNIH) Sarcopenia Project. J Gerontol A Biol Sci Med Sci 2014;69: 576–583
Landi F, Liperoti R, Russo A et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 2012;31: 652–658
Vetrano DL, Landi F, Volpato S et al. Association of sarcopenia with short-and longterm mortality in older adults admitted to acute care wards: results from the CRIME study. J Gerontol A Biol Sci Med Sci 2014;69: 1154–1161
Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med 2014;127: 547–553
Cawthon PM, Fox KM, Gandra SR et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc 2009;57: 1411–1419
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004;52: 80–85
Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res 2016;28: 1047–1060
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39: 412–423
Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Bodysize dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab 2002;282: E132–138
Abe T, Patterson KM, Stover CD, Young KC. Influence of adipose tissue mass on DXA-derived lean soft tissue mass in middle-aged and older women. Age (Dordr) 2015;37: 9741
Loenneke JP, Loprinzi PD, Abe T. The prevalence of sarcopenia before and after correction for DXA-derived fat-free adipose tissue. Eur J Clin Nutr 2016;70: 1458–1460
Boutin RD, Yao L, Canter RJ, Lenchik L. Sarcopenia: Current concepts and imaging implications. AJR Am J Roentgenol 2015;205: W255–266
Nijholt W, Scafoglieri A, Jager-Wittenaar H, Hobbelen JS, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 2017;8: 702–712
Abe T, Loenneke JP, Thiebaud RS. The use of ultrasound for the estimation of muscle mass: One site fits most? J Cachexia Sarcopenia Muscle 2018;9: 213–214
Sanada K, Kearns CF, Midorikawa T, Abe T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol 2006;96: 24–31
Abe T, Thiebaud RS, Loenneke JP, Young KC. Prediction and validation of DXAderived appendicular lean soft tissue mass by ultrasound in older adults. Age (Dordr) 2015;37: 114
Abe T, Fujita E, Thiebaud RS, Loenneke JP, Akamine T. Ultrasound-derived muscle thickness is a powerful predictor for estimating DXA-derived appendicular lean mass in Japanese older adults. Ultrasound Med Biol 2016;42: 2341–2344
Abe T, Kondo M, Kawakami Y, Fukunaga T. Prediction equations for body composition of Japanese adults by B-mode ultrasound. Am J Hum Biol 1994;6: 161–70
Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and SEM. J Strength Cond Res 2005;19: 231–240
Abe T, Counts BR, Barnett BE, Dankel SJ, Lee K, Loenneke JP. Associations between handgrip strength and ultrasound-measured muscle thickness of the hand and forearm in young men and women. Ultrasound Med Biol 2015;41: 2125–2130
Abe T, Loenneke JP, Thiebaud RS, Fukunaga T. Age-related site-specific muscle wasting of upper and lower extremities and trunk in Japanese men and women. Age (Dordr) 2014;36: 813–821
Abe T, Patterson KM, Stover CD et al. Site-specific thigh muscle loss as an independent phenomenon for age-related muscle loss in middle-aged and older men and women. Age (Dordr) 2014;36: 9634
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://doi.org/www.R-project.org/2017.
Chen LK, Liu LK, Woo J et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15: 95–101
Chen Z, Wang ZM, Lohman T et al. Dual-energy X-ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr 2007;137: 2775–2780
Levine JA, Abboud L, Barry M, Reed JE, Sheedy PF, Jensen MD. Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol 2000;88: 452–456
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abe, T., Thiebaud, R.S., Loenneke, J.P. et al. DXA-Rectified Appendicular Lean Mass: Development of Ultrasound Prediction Models in Older Adults. J Nutr Health Aging 22, 1080–1085 (2018). https://doi.org/10.1007/s12603-018-1053-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-018-1053-1