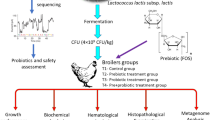
Abstract
Enterococcus faecium, Bifidobacterium, and Pediococcus acidilactici, as intestinal probiotics, have been proved to play a positive role in treating intestinal diseases, promoting growth and immune regulation in poultry. The aim of this study was to evaluate the effect of compound probiotics on growth performance, digestive enzyme activity, intestinal microbiome characteristics, as well as intestinal morphology in broiler chickens. Treatment diets with chlortetracycline and compound probiotics were used for two groups of sixty broilers each throughout the feeding process. Another group was fed the basal diet. The BW (2589.41 ± 13.10 g vs 2422.50 ± 19.08 g) and ADG (60.57 ± 0.31 g vs 56.60 ± 0.45 g) of the compound probiotics added feed treatment group were significantly increased, and the FCR was significantly decreased (P < 0.05). The supplementation of a compound probiotics enhanced the abundance of beneficial bacteria such as Lactobacillus, Faecalibacterium, and norank_f_norank_o_Clostridia_vadinBB60_group (P < 0.05), and modulated the cecal microbiota structure, thereby promoting the production of short-chain fatty acids (SCFAs) and elevating their levels (P < 0.05), particularly propionic and butyric acids. Furthermore, the administration of the compound probiotics supplements significantly enhanced the villi height, V/C ratio, and reduced the crypt depth (P < 0.05). In addition, the activity of digestive enzymes in the duodenum and jejunum was elevated (P < 0.05). Collectively, the selected compound probiotics supplemented in this experiment have demonstrated efficacy, warranting further application in practical production settings as a viable alternative to antibiotics, thereby facilitating efficient production and promoting gastrointestinal health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the years, chicken production increased, making great contributions to human protein supply and becoming an indispensable source of animal protein [1]. The high-quality and efficiency broiler products is inseparable from the health of animals in the process of farming. Antibiotics have been used in the poultry industry for decades to combat infectious pathologies and perform preventative treatment. As additives in animal feed, antibiotics act excellently on improving feed efficiency and production performance [2,3,4]. For example, chlortetracycline is a typical antibiotic capable of fighting Gram-positive and Gram-negative bacteria, as well as some protozoa and mycobacteria. It is commonly used as a feed additive to promote growth and provide a good disease control [5].
Antibiotic drugs are commonly used in poultry for three purposes: (1) therapeutic use, where animals (individual or small groups) are used of high doses of antibiotics over a relatively short period of time; (2) prophylactic use, in which animals are exposed to moderate doses of antibiotics for longer time duration; and (3) growth promoting use, that is, subtherapeutic doses of antibiotics promote growth [4]. However, excessive and blind misuse of antibiotics in production and treatment for the purpose of growth promotion has accelerated the development of antibiotic resistance and the accumulation of residues in animal products.. Moreover, antibiotic use disrupts the intestinal microbiome and intestinal barrier, resulting in impaired intestinal immune function and threatening the health of animals themselves [6, 7]. In the process of the use of antibiotics in broiler breeding, only a small part of antibiotics can be utilized by the body to produce corresponding effects, while most of the rest antibiotics may accumulate in the body of broiler and induce antibiotic resistance genes with microbial flora [8, 9]. Therefore, producing broilers without antibiotics is critical to saving poultry and public health in the fight against antimicrobial resistance. In recent years, the use of feed additives to replace the addition of antibiotics has become a hot topic, including probiotics, prebiotics, organic acids, exogenous enzymes, and plant extracts [10, 11].
Increasing evidence has shown that intestinal microbiota play important role in keeping host health, suggesting the potential of manipulation of intestinal microbiota through probiotics practicing to maintain animal health [12]. Probiotics and antimicrobial peptides (AMPs) are two promising approaches that have shown potential benefits in various diseases. Probiotics are live microorganisms that confer health benefits to the host when administered in adequate amounts [13]. AMPs, usually produced with probiotic bacteria, are short amino acid sequences that have broad-spectrum activity against bacteria, fungi, viruses, and parasites. Both probiotics and AMPs can modulate the host immune system, inhibit the growth and adhesion of pathogens, disrupt biofilms, and enhance intestinal barrier function. Some studies have shown that intestinal symbiotic bacteria could induce AMPs and the presence of antibiotics such as penicillin could decrease the expression of AMPs genes [14]. The use of AMPs is more inclined to directly inhibit the growth of harmful bacteria and has a strong bactericidal effect. One of the advantages of using AMPs is that microorganisms cannot develop a resistance to them; therefore, they may represent an important tool in the treatment of MDR bacterial infections [15]. Unfortunately, there are some drawbacks for the immediate use of natural AMPs in clinical practice as they are susceptible to proteolytic degradation and have low oral bioavailability [16]. Therefore, in broiler production, probiotics are more universal, can provide multiple benefits, and play an important role in regulating intestinal microbial communities, promoting nutrient absorption and enhancing immunity.
Enterococcus faecium, as a lactic acid bacterium (LAB), can effectively improve the growth performance of animals, reduce mortality, improve intestinal morphology, beneficially regulate the intestinal microbiome of broilers, and can improve Phosphorus absorption and bone mineralization, promote healthy growth of animals, and prevent the occurrence of diseases [17, 18]. In the same way, Bifidobacterium is used as a substitute for growth-promoting antibiotics, which can effectively improve the body weight and feed conversion rate of broilers, thereby improving the growth performance of broilers [19]. It has been found that the combination of oligosaccharides and Bifidobacterium, as a superior product to replace antibiotics in broiler production, can not only improve growth efficiency, but also establish a favorable intestinal microbiome composition, thereby giving the host intestinal health benefits [20]. Pediococcus acidilactici, one of the probiotics commonly used in broiler production, showed an important advantage in the production process is that it can balance the ratio of Firmicutes to Bacteroidota to modulate intestinal microbiome homeostasis and reduce the abundance of pathogenic enterobacteria [21, 22].
From what has been discussed above, these studies strongly showed that adding probiotics to the diet can improve nutrient utilization and promote growth. Nevertheless, less research has focused on the role of compound probiotics in broiler production. Therefore, the purpose of this experiment was to investigate the effects of compound probiotics additives composed of Enterococcus faecium, Bifidobacterium and Pediococcus acidilactici on growth performance, digestive enzyme activity, short-chain fatty acids, intestinal flora, and intestinal characteristics of broiler chickens.
Materials and Methods
Compound Probiotics and Antibiotic
The compound probiotics supplement (DSM Singapore Industrial Pte. Ltd.) was composed of three strains of Enterococcus faecium, Bifidobacterium, and Pediococcus acidilactici, by using oligosaccharides and inulin as carriers. The compound probiotics supplemented to diet at a level of 1000 mg/kg, in which the strain dose of Enterococcus faecium, Bifidobacterium, and Pediococcus acidilactici was 2 × 1010 CFU/g, 1 × 1010 CFU/g, and 3 × 109 CFU/g, respectively. The addition amount of chlortetracycline (Wuhan Ammunition Life-tech Co., Ltd., Wuhan, China) was 80 mg/kg.
Broiler, Diet, and Housing Management
A total of 180 1-day-old Arbor Acres (AA) broilers were weighted and divided into three groups as control group (CON), chlortetracycline antibiotics group (CTC), and compound probiotics supplementation group (PSM) in a completely randomized design. There were ten broilers assigned in a cage, and six replicates of cages in each treatment. The corn-based basal diets were formulated for grower from day 1 to 21 and finisher from day 22 to 42 (Table 1). All diets met or exceeded nutrient requirements of broilers according to the provisions of National Research Council (NRC, 1994). Feed and water were supplied ad libitum.
Broilers were kept in three-layer cages with length, width and height of 150 cm × 60 cm × 70 cm for one cell. The initial room temperature was 33 ℃ in the first 3 days and gradually decreased to 24 ℃ at 28 days of age. After that, the temperature was kept at 22 ~ 24 ℃ until the end of the experiment. The light schedule was a cycle of 23-h light and 1-h darkness throughout the entire experiment.
Growth Performance and Sample Collection
Broiler body weight and their feed in each replicate cage were evaluated weekly (D1, D7, D14, D21, D28, D35, and D42). The average body weight (ABW), average daily gain (ADG), and average daily feed intake (ADFI) of each treatment were calculated during the grower (D1–D21), finisher (D21–D42), and overall periods (D1–D42). The ratio between ADFI and ADG (F/G) was also calculated from ADFI and ADG data.
At D21 and D42, one chicken with the body weight close to the average in each cage was euthanized for tissue sample collection. There were six individuals from each treatment. The intestinal tissue of duodenum and jejunum was stored at polyformaldehyde to test intestinal histomorphological analysis, and intestinal contents of duodenum, jejunum, and cecum were collected and stored at −80 ℃ to test digestive enzyme activity, SCFA concentration, and intestinal microbiome.
Digestive Enzyme Activity in Duodenum and Jejunum
The activities of amylase, lipase, trypsin, and chymotrypsin in the intestinal contents were measured with commercial kits (Nanjing Jian Cheng Bioengineering Institute, Nanjing, China) following the instructions of the manufacturer. To quantify digestive enzyme activity, 100 mg of each intestinal content was removed into a centrifugal tube and the supernatant was harvested after centrifugation at 4000 rpm/min for 10 min at 4 ℃. The obtained supernatant is performed according to the instructions of the digestive enzyme kit to be measured.
SCFA Concentration in Cecum
The short-chain fat acids (SCFA, including acetic acid, propanoic acid, isobutyric acid, butyric acid, isovaleric acid and valeric acid) in cecal digest were determined by gas chromatography (GC) method according to the described of Dang et al. [23]. SCFAs in the digest were extracted by ultrapure water at 10,000 × g centrifugation, and 25% metaphosphoric acid was mixed with the extracts at a ratio of 1:9. After centrifugation of 12,000 × g, the mixture was passed through the 0.45-µm Milled-LG filter (Millipore, Billerica, MA, USA) and subjected for SCFA analysis with the Agilent 7890 N gas chromatograph (Agilent, Santa Clara, CA, USA).
Intestinal Histomorphological Analysis
Hematoxylin and eosin (H&E) staining was used to analyze intestinal histomorphology including villus height, crypt depth, and V/C ratio cited as described by Yin et al. [24]. The duodenum and jejunum samples were dehydrated, embedded in paraffin, and then sliced at 5 µm thickness. The samples were stained with H&E and observed on a Leica DM2000 light microscope (Leica Microsystems, Wetzlar, Germany). The images were analyzed with ImageJ version 1.8 software (National Institutes of Health, MD, USA). Two replicates of complete villus and crypt from each histological section were selected for measurement.
DNA Extraction, Amplification, and Sequencing
According to the manufacturer’s instructions, the bacterial DNA of cecal digest samples was extracted with a PowerSoil DNA Isolation Kit (MoBio Laboratories, Carlsbad, CA, USA). The V3-V4 regions of the 16S rRNA gene were amplified by an ABI GeneAmp® 9700 PCR thermocycler (ABI, Foster, CA, USA). The primers were 338F (5ʹ-ACTCCTACGGGAGGCAGCAG-3ʹ) and 806R (5ʹ-GGACTACHVGGGTWTCTAAT-3ʹ). PCR amplification reactions were triplicated and then purified by AxyPrep DNA Gel Extraction Kit (Axygen Bio-sciences, Union City, CA, USA). Purified amplicons were pooled in equimolar and paired end sequenced on an Illumina MiSeq platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China), as previously described [25]. The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database.
Raw fastq files were demultiplexed, quality-filtered by QIIME (version 1.70). Operational taxonomic units (OTUs) were clustered using a 97% similarity cutoff with UPARSE (version 7.1), and chimeric sequences were removed using UCHIME (version 7.1). OUT representative sequence was obtained based on RDP classifier [26].
Statistical Analysis
The statistical analyses were performed using SPSS statistical program (IBM, version 20, Chicago, IL, USA, 2011). The data were analyzed using the general linear model (GLM) procedure. Analysis of variance (ANOVA) tests were used for analyses of variance accompanied by Duncan’s multiple range test to detect the differences between the treatments. Moreover, ANOVA with repeated measurements was applied for BW, AD, feed intake, and FCR results. The results are presented as mean ± standard error of the mean. Probability values less than 0.05 (P < 0.05) were considered significant.
Results
Growth Performance
The effects of compound probiotics supplementation on growth performance of broilers are shown in Table 2. In all groups, there was no discernible variation in the initial body weight of broilers. Broilers fed with the diet of chlortetracycline and compound probiotics showed better growth performance compared to the CON group, which was characterized by the significant increase in BW and ADG, reduced FCR at phase 1 and phase 2, and all growing cycle (P < 0.05) and feed intake were not affected by supplementation (P > 0.05). For all phases, PSM group had no significant differences between CTC group on BW, ADG feed intake, and FCR. The surviving rates were 100% in all treatments. Altogether, these findings demonstrated that compound probiotics had effective effects on the growth performance of broilers.
Digestive Enzymes Activity in Duodenum and Jejunum
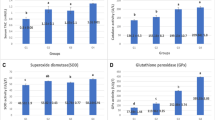
The activities of digestive enzymes (amylase, lipase, trypsin and chymotrypsin) of broilers intestinal contents are presented in Fig. 1. According to the findings, CTC group and PSM group significantly increased the activity of digestive enzymes in the duodenum compared to the CON group (P < 0.01; Fig. 1A–D). Furthermore, at D21, the activity of amylase, lipase, trypsin, and chymotrypsin in PSM group was considerably higher than that in CTC group (P < 0.01). However, there was no distinction in the activity of these four digestive enzymes between PSM group and CTC group at D42 (P > 0.05). For jejunum (Fig. 1E–H), at D21, there was no significant difference in the digestive enzyme activities between CON group, CTC group, and PSM group (P > 0.05), whereas, at D42, digestive enzyme activities in PSM group were the highest, followed by these in CTC group, which were noticeably higher than these in CON group, and reached significant level (P < 0.01). These results indicated that compound probiotics was effective in improving the intestinal digestive enzyme activity of broilers, and the effect was better than chlortetracycline.
Effects of compound probiotics on digestive enzyme activity in duodenum and jejunum of broilers. A Amylase in duodenum; B lipase in duodenum; C trypsin in duodenum; D chymotrypsin in duodenum; E amylase in jejunum; F lipase in jejunum; G trypsin in jejunum; H chymotrypsin in jejunum (n = 6/group). Data is presented as the mean ± SEM. Signification is presented as *P < 0.05, **P < 0.01, and ***P < 0.001
Composition and Variation of Microbiota in Cecum
To assess the effect of compound probiotics on intestinal microbiota, we profiled the cecal microbial community composition and structure by using 16S rRNA gene sequencing. After applying the previously reported method to remove disqualified sequences, the 18 samples from three groups at D21 were flattened with 27,370 valid sequences and 45,601 valid sequences at D42. The rarefaction curves for all samples approached the plateau indicated that the sequencing depth was sufficient to capture the majority of operational units present in our samples (Fig. 2A and B). Alpha diversity (Chao and Shannon index) revealed the intestinal microbial flora diversity of CTC group and PSM group did not show significantly change compared with CON group (Fig. 2C and D, P > 0.05). Furthermore, there were 394, 384, and 384 operational taxonomic units (OTUs) obtained from CON, CTC, and PSM groups at D21, respectively, of which 342 OTUs were common among the three experimental groups. Similarly, 517, 484, and 499 OTUs were classified in CON, CTC, and PSM groups at D42 with 458 common OTUs (Fig. 2E and F). Principal component analysis (PCA) is shown in Fig. 2G and H. At both D21 and D42, PSM group had a strong effect on the beta diversity of the intestinal microbiota in broilers. These results indicated that the compound probiotics supplementation was able to change bacterial community structure in cecum.
Effects of compound probiotics on microbial community composition. A, B Rarefaction curves tended to reach the plateau; C the alpha-diversity of cecum microbiota in chyme of Chao index; D the alpha diversity of cecum microbiota in chyme of Shannon index; E VENN diagram at D21; F VENN diagram at D42; G principal component analysis (PCA) at D21; H principal component analysis (PCA) at D42. Data is presented as the mean ± SEM, n = 6
In terms of the composition of microbial community, Firmicutes was the most abundant phylum at both D21- and D42-associated communities in CON, CTC, and PSM groups. Other advantage phyla were Bacteroidota, Proteobacteria, and Actinobacteriota (Fig. 3A). There was no significant difference in the relative abundance of microbiota at the phylum level (Fig. 3B, P > 0.05). Moreover, at the genus level, the composition of the intestinal microbiota included three major genera, unclassified_f_Lachnospiraceae, Alistipes, and Ruminococcus_torques_group at D21 and unclassified_f_Lachnospiraceae, Faecalibacterium, and Barnesiella at D42 (Fig. 3C). In the next step, we compared the top 15 genera in relative abundance among the three groups (Fig. 3D). At D21, the relative abundances of Lactobacillus and Faecalibacterium were significantly increased in PSM group compared with CON group (P < 0.05). However, the relative abundances of Alistipes and Sellimonas in PSM group were significantly decreased in CON group (P < 0.05). The relative abundances of Faecalibacterium and Alistipes in PSM group were greater than CON group and Barnesiella and norank_f_norank_o_Clostridia_vadinBB60_group were lower than CON group at D42 (P < 0.05). In the case of the CTC group, at D21, Lactobacillus, Faecalibacterium, and norank_f_norank_o_Clostridia_UCG-014 have increased, while Alistipes, Ruminococcus_torques_group, and Blautia have reduced compared with CON group (P < 0.05). And for D42, only Faecalibacterium has increased, while Alistipes and norank_f_norank_o_Clostridia_vandinBB60_group have decreased (P < 0.05). All these findings demonstrated that the addition of chlortetracycline and compound probiotics had no effect on the abundance of intestinal microbiota, but alter their composition in broilers.
Effects of compound probiotics on the microbiota at the phylum and genus level. A The relative abundance of bacterial at the phylum level; B the top 2 phylum statistical comparison of the relative abundance in three groups; C the relative abundance of bacterial at the genus level; D the top 15 genus statistical comparison of the relative abundance in three groups
SCFA Concentration in Cecum
In order to assess the impact of compound probiotics on the metabolites of the intestinal microbiota, an analysis was conducted on the concentration of short-chain fatty acids (SCFAs) in cecal chyme, as depicted in Fig. 4. At D21, the findings indicated a significant increase in the concentration of acetic acid, butyric acid, and total SCFAs in both CTC group and PSM group when compared to CON group (P < 0.01). And there was no significant difference between these three groups in terms of the concentration of propanoic acid, isobutyric acid, isovaleric acid, and valeric acid (P > 0.05). While at D42, the results showed that compared with CON group, the concentration of acetic acid, propanoic acid, isobutyric acid, isovaleric acid, valeric acid, and total SCFAs was dramatically reduced in CTC group and PSM group (P < 0.01). Butyric acid was the only metabolite that did not significantly change between the three groups (P > 0.05). Additionally, the fraction of butyric acid in PSM group was increased despite a reduction in total SCFAs compared with CON group. In general, compound probiotics had beneficial effects on metabolites SCFAs, either by increasing the concentration of total SCFAs or by increasing the concentration of energy-supplying butyric acid.
Effects of compound probiotics on SCFAs concentration. A SCFA concentration at D21; B the proportion of each SCFAs at D21; C SCFA concentration at D42; D the proportion of each SCFAs at D42 (n = 6/group). Data is presented as the mean ± SEM. Signification is presented as *P < 0.05, **P < 0.01, and ***P < 0.001
The Intestinal Morphology in Duodenum and Jejunum
As illustrated by H&E staining of the intestinal morphology and its measurement parameters (Figs. 5 and 6), the duodenum and jejunum of the broilers in CON group were integral, and composed of slender villi and complete crypts. Additionally, the broilers in PSM group also had intact lumens and unbroken villi. Inversely, in CTC group, the villi were tall but sparse, with occurrences of fractures and breakage in the lumen. Besides, the crypts were irregular compared with CON group. In duodenum, villus height and V/C ratio of the broilers in PSM group were increased and crypt depth was reduced compared with CON group and CTC group at D21 (P < 0.01). At D42, the villus height and V/C ratio of PSM group were significantly higher than CON group (P < 0.01); yet, villus height, crypt depth, and V/C ratio were essentially the same between PSM group and CTC group (P > 0.05). In the case of jejunal morphology, broilers in PSM group as well had higher villus height, lower crypt depth, and higher V/C ratio (P < 0.01). Nevertheless, the difference is that higher villus, deeper crypt, and decreasing V/C ratio appeared in PSM group compared with CTC group at D42 (P < 0.01). The results declared that both chlortetracycline and compound probiotics had positive effects on the intestinal morphology of broilers, and compound probiotics has a stronger positive effect than chlortetracycline.
Effects of compound probiotics on intestinal morphology in duodenum of broilers. A Villus height in duodenum; B crypt depth in duodenum; C The ratio of villus height to crypt depth (V/C ratio) in duodenum; D The staining profiles by H&E, scale bars 500 μm (n = 12/group). Data is presented as the mean ± SEM. Signification is presented as *P < 0.05, **P < 0.01, and ***P < 0.001
Effects of compound probiotics on intestinal morphology in jejunum of broilers. A Villus height in duodenum; B crypt depth in duodenum; C the ratio of villus height to crypt depth (V/C ratio) in duodenum; D The staining profiles by H&E, scale bars 500 μm (n = 12/group). Data is presented as the mean ± SEM. Signification is presented as *P < 0.05, **P < 0.01, and ***P < 0.001
Discussion
Probiotics are the most commonly consumed food additives, commonly found in yogurt, cheese, ice cream, snacks, and nutrition bars, and are widely supported by gastroenterologists [27, 28]. Despite the widespread popularity of probiotics, the findings from extensive research conducted over several decades on the effectiveness of probiotics in disease treatment and prevention often yield conflicting conclusions, leading to ongoing debates and discussions [29, 30]. In broiler production, looking through a large number of probiotic use cases, it is found that the addition of probiotics is an important means to improve the production efficiency of broilers and inhibit the occurrence of diseases. Therefore, reasonable and moderate use of appropriate probiotics to guide the production of broilers has a very important industrial significance.
Enterococcus faecium, Bifidobacterium, and Pediococcus acidilactici are considered promising probiotics for maintaining intestinal health and improving production performance, in broiler production [31,32,33]. It was reported that diet supplement Enterococcus faecium remarkably increased ADG (quadratically) and FCR (linearly) in different change forms during the whole feeding process [34]. In addition, body weight gain and FCR of broilers fed Bifidobacterium were better than those fed control diets [35]. In terms of Pediococcus acidilactici, Wu et al. [36] found that after supplementation, BWG and FI did not increase, but FCR decreased. In this study, the performance of compound probiotics supplementation group was consistent with that of chlortetracycline supplementation group, which was significantly better than the control group. The chlortetracycline and PSM compound probiotics significantly increased BW and ADG and decreased FCR of broilers during the whole production process. However, the mechanism of compound probiotics improving broiler performance is very different from chlortetracycline.
In the present study, feeding with compound probiotics significantly increased the activities of amylase, lipase, trypsin, and chymotrypsin in the duodenum during the whole period. Feeding with compound probiotics had no significant difference in jejunal digestive enzyme activity at D21 compared with the CON group, but significantly increased at D42. Meanwhile, BW and FCR of broilers from PSM group were also improved. These endogenous amylase, lipase, and protease are important for the decomposition, digestion, and absorption of crude proteins, lipids, and carbohydrates from macromolecules to amino acids, triglycerides, and glucose in broilers. Therefore, the increase level of digestive enzyme activities by compound probiotics supplementation could be the reasons for improving the nutrient digestibility and production performance of broilers. It has now been found that the increased activity of digestive enzymes may be caused by the secretion of probiotics digestive enzymes or by the strengthened secretion from cells stimulated by these probiotics, or a combination of these two factors, thereby promoting the secretion of digestive enzymes [37]. Moreover, Enterococcus faecium and Pediococcus acidilactici could also enhance the development and nutritional function of duodenum and jejunum, which can also encourage the improvement of intestinal enzyme activity [18, 22]. In our study, there was a significant improvement in the intestinal morphological characteristics of broilers after feeding probiotic complexes to enhance the activity of intestinal digestive enzymes. It can be concluded that the activity of intestinal digestive enzymes was affected by exogenous probiotics, and compound probiotics product enhanced the intestinal digestive enzymes activity, which can improve the digestion and absorption rate of broiler chickens, thereby promoting the rapid growth of broilers.
The cecum is the predominant site of fermentation in the digestive tract of broilers and harbors a diverse microbial community. Microorganisms of the cecum profile were directly linked to animal health [38]. These results might be explained by the higher level abundance of Firmicutes and lower levels of Bacteroidetes, which were previously assumed to be linked to weight gain [39]. The present study reported that broilers fed with compound probiotics increased the ratio of Firmicutes to Bacteroidota to beneficially modulate the intestinal flora structure. High proportion of Firmicutes to Bacteroidota of intestinal microbes of broilers was a response to the event of the high-fiber diet resources, and it could help broilers to obtain more energy from dietary food [40]. In addition, the difference in nutrient absorption caused by intestinal permeability and intestinal wall thickness, the influence of microorganisms on material metabolism, and their own metabolites may also affect body weight. Many previous studies confirmed that Enterococcus faecium, Bifidobacterium, and Pediococcus acidilactici can improve microbial composition, microbial metabolites, and intestinal morphometry [18, 19, 21]. Meanwhile, some studies found that the addition of probiotics had neutral or negative effects on growth performance and intestinal microorganisms of broilers, like that the addition of B. subtilis and E. faecium will destroy the original microbial structural balance and lead to a decrease in the average weight gain of broilers [41]. The reason for this result may be that different types of microorganisms have different effects on intestinal health or that microorganisms consume too much energy from diet, which brings negative effects on production performance. In conclusion, the compound probiotics selected in this experiment are effective on the intestinal microbial balance of broilers and can be further applied in production practice to replace antibiotics to achieve efficient production and animal health.
In recent years, as an important derived metabolite of the intestinal microflora, SCFAs have drawn greater attention [42]. SCFAs can promote intestinal health, especially, acetic acid, propanoic acid, and butyric acid can be directly absorbed as a nutrient and help maintain intestinal mucosal integrity, they became one of the most noteworthy SCFAs [43, 44]. In this study, we investigated intestinal microbial metabolites and found that there was an improvement in the concentration of acetic acid, butyric acid and total SCFAs of broilers fed compound probiotics supplementation diets at D21. These results were attributed to the increased abundance of SCFA producing bacteria, which is critical for SCFA production, by the supplementation of compound probiotics. Among the top 15 genera, Ruminococcus_torques_group, Lactobacillus, Faecalibacterium, norank_f_norank_o_Clostrida_UCG-014, norank_f_norank_o_Clostridia_vadinBB60_group, and Butyricicoccus have been documented as a bacterium that exerts SCFA-producing capabilities [45,46,47]. In agreement with our studies, previous studies reported that broilers fed probiotics supplementation diets had higher SCFA production at D21 cecum compared with broilers fed diet without probiotics supplementation [48]. In this study, the supplementation of compound probiotics increased the abundance of SCFA-producers such as Lactobacillus, Faecalibacterium, and norank_f_norank_o_Clostridia_vadinBB60_group, consequently resulting in an increment in SCFA levels. Moreover, intestinal microbiota primarily produces SCFAs by fermenting monosaccharides derived from nutrients and mucins, with acetate and butyrate being promoted mainly by bacteria such as Ruminococcus spp. and Lactobacillus spp. [49]. This also explains the higher concentrations of cecal acetate and total SCFA in compound probiotics supplementation. Furthermore, some studies have revealed that SCFAs can increase intestinal acidity, such as butyrate and propionate, which have inhibitory effects on foodborne pathogen. However, the results of studies on SCFA levels in cecal digest of broilers at different days age are inconsistent. Czerwiński et al. [50] added probiotics to broiler diets and found that the concentrations of acetic acid, butyric acid as well as total SCFAs in the intestine were significantly reduced following addition of the dietary probiotic. Similarly, within this study, while an increment in SCFA-producers was observed at D42, there was a concurrent decline in the concentration of total SCFAs in the PSM group. Since the concentration of SCFAs in cecum is affected by many factors [51], the physiological role of SCFAs in different growth stages needs to be further studied.
Intestinal morphology is the most intuitive manifestation of the health and integrity of the digestive tract, including villus height, crypt depth, and V/C ratio. Increased villus height indicates a larger area for nutrient absorption in the intestine and more mature enterocytes accumulating, which representing enhanced absorptive capacity [52]. Shorter villi and deeper crypts were observed, suggesting reduced digestive and absorptive function. The ratio of V/C can reflect the turnover rate of intestinal cells [53]. Consistent with our findings, numerous studies have shown that dietary supplementation with probiotics can affect intestinal morphological parameters [54]. In this study, we demonstrated that the PSM group showed significantly increased villus height and V/C ratio, and decrease crypt depth in ileum at D21. This findings were in agreement with the results of Xie et al. [54], who showed that the addition of probiotics improved the intestinal morphology of the experimental chickens, including increased villus height and V/C ratio, and decreased crypt depth. Huang et al. [18] also found that the addition of Faecalibacterium improved intestinal histomorphology, increasing the V/C ratio and villus height in infected birds. Improvements in intestinal morphology may be indirectly due to improved nutritional status (SCFAs, amino acids, and others) causing fermentation of metabolites [55]. The administration of compound probiotics has been shown to enhance the abundance of beneficial bacteria, including Lactobacillus, Faecalibacterium, and norank_f_norank_o_Clostridia_vadinBB60_group, thereby facilitating improved nutrient digestion and absorption. Simultaneously, compound probiotics increased the production of SCFAs, particularly propionic acid and butyric acid, which were conducive to intestinal growth and development, enhancing intestinal health and feed conversion efficiency in broiler chickens. Such results revealed that compound probiotics had an improved effect on intestinal morphology, which may help to promote the absorption of nutrients in broilers, thereby enhancing growth performance.
As normal inhabitants of diverse ecosystems, including the gastrointestinal tract, Enterococcus faecium, Bifidobacterium, and Pediococcus acidilactici can be considered critical to intestinal microecology. Enterococcus faecium does not colonize permanently in animals; often, temporary colonization occurs after administration with the decreasing number of Enterococcus faecium CFU over time [56]. Therefore, this study selected the supplementation of compound probiotics for the whole production of broilers and found that after the supplementation, the activity of digestive enzymes in broilers was significantly increased and generation direction of SCFAs, microbiota structure, and intestinal morphology was significantly improved. Research into the beneficial properties of compound probiotics has unveiled a range of mechanisms, including the production of bioactive molecules, such as SCFAs, and digestive enzymes. One of the reasons for the physiological effect of compound probiotics on the growth performance of broilers is to improve the activity of a series of digestive enzymes such as amylase, lipase, trypsin and chymotrypsin, which is a direct reflection of the improvement of intestinal nutrient metabolism ability of broilers. Acetic acid can be used by butyric acid producing bacteria in the intestine, such as Faecalibacterium, to produce butyric acid [57]. Butyric acid is used by intestinal epithelial cells as an energy source and is involved in a variety of physiological functions, including intestinal barrier function and immune function [58, 59]. In the intestine, Bifidobacterium metabolizes carbohydrates to SCFAs, acetate, and lactate [60]. Although bifidobacteria do not directly synthesize butyric acid, their production of acetic acid may influence the activity and composition of other members of the gut microbiota that produce butyric acid, thereby stimulating the secondary butyric acid effect [61, 62]. In addition, this study also found that the supplementation of compound probiotics directly increased the relative abundance of intestinal Faecalibacterium and drive SCFA optimization in the direction of butyric acid generation, which further explained the reasons for the improvement of intestinal morphology and the improvement of feed efficiency. Although the compound probiotics in this study had good effects on growth performance, intestinal microbiota, and intestinal tissue morphology of broilers, and were consistent with the results of current high-quality studies, there were still contrary conclusions, and the differences may be attributed to the experimental design of different studies, different measurement parameters, and experimental conditions. Probiotics secrete many substances (metabolites), and there are many possible pathways to action, most of which have not been fully elucidated [63]. The complex ratio of compound probiotics and the difference in the proportion of active ingredients are the important reasons for the differences in experimental results. Secondly, “strain difference” — in recent years, human microecological studies have emphasized that the research needs to be refined to the “strain level.” The probiotic species and strains used in experimental research are different, and the combination of multiple strains makes it more difficult to elucidate the health effects of probiotics [29]. In addition, the standardization of animal models and production of probiotics also affect the research and clinical application of probiotics. In the future, more production trials of compound probiotics and more optimization and standardization of the ratio will help provide a more powerful basis for more widespread application in the use of antibiotic replacement.
Conclusion
In summary, supplementation of compound probiotics based on Enterococcus faecium, Bifidobacterium, and Pediococcus acidilactici in diets enhanced growth performance of broiler chickens, manifested by increasing BW, ADG, and reducing FCR. During this process, compound probiotics enhanced the relative abundance of beneficial bacteria Lactobacillus, Faecalibacterium, and norank_f_norank_o_Clostridia_vadinBB60_group, thereby optimizing the intestinal microbiota. The increase in SCFAs produced by probiotics is beneficial to increase villus height, decrease crypt depth, and increase villus/crypt ratio. In addition, the stimulation of the well-developed intestine by probiotics promotes the enhancement of intestinal digestive enzyme activity. Therefore, the use of compound probiotics improved the growth performance of broilers by regulating the intestinal microbial community to promote early intestinal development.
Data Availability
All 16S rRNA Illumina amplicon sequencing data provided in this study can be publicly obtained in the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) under the accession number SRP PRJNA947554. The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Fancher CA, Zhang L, Kiess AS, Adhikari PA, Dinh TTN, Sukumaran AT (2020) Avian pathogenic Escherichia coli and Clostridium perfringens: challenges in no antibiotics ever broiler production and potential solutions. Microorganisms 8:1533. https://doi.org/10.3390/microorganisms8101533
Gaskins HR, Collier CT, Anderson DB (2002) Antibiotics as growth promotants: mode of action. Anim Biotechnol 13:29–42. https://doi.org/10.1081/ABIO-120005768
Hao H, Sander P, Iqbal Z, Wang Y, Cheng G, Yuan Z (2016) The risk of some veterinary antimicrobial agents on public health associated with antimicrobial resistance and their molecular basis. Front Microbiol 7:1626. https://doi.org/10.3389/fmicb.2016.01626
Ferdous MRA, Ahmed MR, Khan S et al (2020) Effect of discriminate and indiscriminate use of oxytetracycline on residual status in broiler soft tissues. Vet Worl 1:61–67. https://doi.org/10.14202/vetworld.2020.61-67
Cornejo J, Yevenes K, Avello C et al (2018) Determination of chlortetracycline residues, antimicrobial activity and presence of resistance genes in droppings of experimentally treated broiler chickens. Molecules 23:1264. https://doi.org/10.3390/molecules23061264
Zhang S, Zhong R, Han H et al (2020) Short-term lincomycin exposure depletion of murine microbiota affects short-chain fatty acids and intestinal morphology and immunity. Antibiotics (Basel) 9:907. https://doi.org/10.3390/antibiotics9120907
Graversen KB, Bahl MI, Larsen JM, Ballegaard AR, Licht TR, Bogh KL (2020) Short-term amoxicillin-induced perturbation of the gut microbiota promotes acute intestinal immune regulation in brown Norway rats. Front Microbiol 11:496. https://doi.org/10.3389/fmicb.2020.00496
Tasho RP, Cho JY (2016) Veterinary antibiotics in animal waste, its distribution in soil and uptake by plants: a review. Sci Total Environ 563–564:366–376. https://doi.org/10.1016/j.scitotenv.2016.04.140
Haque MH, Sarker S, Islam M et al (2020) Sustainable antibiotic-free broiler meat production: current trends, challenges, and possibilities in a developing country perspective. Biology (Basel) 9:411. https://doi.org/10.3390/biology9110411
Kiarie E, Romero LF, Nyachoti CM (2013) The role of added feed enzymes in promoting gut health in swine and poultry. Nutr Res Rev 26:71–88. https://doi.org/10.1017/S0954422413000048
Wu W, Xiao Z, An W, Dong Y, Zhang B (2018) Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS ONE 13:e0197762. https://doi.org/10.1371/journal.pone.0197762
Jain M, Stitt G, Son L, Enioutina EY (2023) Probiotics and their bioproducts: a promising approach for targeting methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Microorganisms 11(10):2393. https://doi.org/10.3390/microorganisms11102393
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Baindara P, Chakraborty R, Holliday ZM, Mandal SM, Schrum AG (2021) Oral probiotics in coronavirus disease 2019: connecting the gut-lung axis to viral pathogenesis, inflammation, secondary infection and clinical trials. New Microbes New Infect 40:100837. https://doi.org/10.1016/j.nmni.2021.100837
Zimina M, Babich O, Prosekov A, Sukhikh S, Ivanova S, Shevchenko M, Noskova S (2020) Overview of global trends in classification, methods of preparation and application of bacteriocins. Antibiotics (Basel) 9(9):553. https://doi.org/10.3390/antibiotics9090553
Mahlapuu M, Björn C, Ekblom J (2020) Antimicrobial peptides as therapeutic agents: opportunities and challenges. Crit Rev Biotechnol 40(7):978–992. https://doi.org/10.1080/07388551.2020.1796576
Champagne CP, Raymond Y, Arcand Y (2017) Effects of production methods and protective ingredients on the viability of probiotic Lactobacillus rhamnosus R0011 in air dried alginate beads. Can J Microbiol 63:35–45. https://doi.org/10.1139/cjm-2016-034
Huang L, Luo L, Zhang Y, Wang Z, Xia Z (2019) Effects of the dietary probiotic, Enterococcus faecium NCIMB11181, on the intestinal barrier and system immune status in Escherichia coli O78- challenged broiler chickens. Probiotics Antimicrob Proteins 11:946–956. https://doi.org/10.1007/s12602-018-9434-7
Dev K, Begum J, Biswas A et al (2021) Hepatic transcriptome analysis reveals altered lipid metabolism and consequent health indices in chicken supplemented with dietary Bifidobacterium bifidum and mannan-oligosaccharides. Sci Rep 11:17895. https://doi.org/10.1038/s41598-021-97467-1
Dev K, Akbar Mir N, Biswas A, Kannoujia J, Begum J, Kant R (2020) Dietary Mannan-oligosaccharides potentiate the beneficial effects of Bifidobacterium bifidum in broiler chicken. Lett Appl Microbiol 71:520–530. https://doi.org/10.1111/lam.13360
Mikulski D, Jankowski J, Mikulska M, Demey V (2020) Effects of dietary probiotic (Pediococcus acidilactici) supplementation on productive performance, egg quality, and body composition in laying hens fed diets varying in energy density. Poult Sci 99:2275–2285. https://doi.org/10.1016/j.psj.2019.11.046
Fu W, Chen C, Xie Q, Gu S, Tao S, Xue W (2022) Pediococcus acidilactici strain alleviates gluten-induced food allergy and regulates gut microbiota in mice. Front Cell Infect Microbiol 12:845142. https://doi.org/10.3389/fcimb.2022.845142
Dang G, Wang W, Zhong R, Wu W, Chen L, Zhang H (2022) Pectin supplement alleviates gut injury potentially through improving gut microbiota community in piglets. Front Microbiol 13:1069694. https://doi.org/10.3389/fmicb.2022.1069694
Yin C, Xia B, Tang S et al (2021) The effect of exogenous bile acids on antioxidant status and gut microbiota in heat-stressed broiler chickens. Front Nutr 8:747136. https://doi.org/10.3389/fnut.2021.747136
Tang S, Zhang S, Zhong R et al (2021) Time-course alterations of gut microbiota and short-chain fatty acids after short-term lincomycin exposure in young swine. Appl Microbiol Biotechnol 105:8441–8456. https://doi.org/10.1007/s00253-021-11627-x
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. https://doi.org/10.1128/AEM.00062-07
Draper K, Ley C, Parsonnet J (2017) Probiotic guidelines and physician practice: a cross-sectional survey and overview of the literature. Benef Microbes 8(4):507–519. https://doi.org/10.3920/BM2016.0146
Williams MD, Ha CY, Ciorba MA (2010) Probiotics as therapy in gastroenterology: a study of physician opinions and recommendations. J Clin Gastroenterol 44(9):631–636. https://doi.org/10.1097/MCG.0b013e3181d47f5b
Suez J, Zmora N, Segal E, Elinav E (2019) The pros, cons, and many unknowns of probiotics. Nat Med 25(5):716–729. https://doi.org/10.1038/s41591-019-0439-x
Freedman SB, Williamson-Urquhart S, Farion KJ et al (2018) Multicenter trial of a combination probiotic for children with gastroenteritis. N Engl J Med 379(21):2015–2026. https://doi.org/10.1056/NEJMoa1802597
Wang W, Cai H, Zhang, et al (2020) Enterococcus faecium modulates the gut microbiota of broilers and enhances phosphorus absorption and utilization. Animals (Basel) 10:1232. https://doi.org/10.3390/ani10071232
Engevik MA, Luk B, Chang-Graham AL et al (2019) Bifidobacterium dentium fortifies the intestinal mucus layer via autophagy and calcium signaling pathways. mBio 10:e01087–19. https://doi.org/10.1128/mBio.01087-19
Pupa P, Apiwatsiri P, Sirichokchatchawan W et al (2021) Use of Lactobacillus plantarum (strains 22F and 25F) and Pediococcus acidilactici (strain 72N) as replacements for antibiotic-growth promotants in pigs. Sci Rep 11:12028. https://doi.org/10.1038/s41598-021-91427-5
Wu Y, Zhen W, Geng Y, Wang Z, Guo Y (2019) Effects of dietary Enterococcus faecium NCIMB 11181 supplementation on growth performance and cellular and humoral immune responses in broiler chickens. Poult Sci 98:150–163. https://doi.org/10.3382/ps/pey368
Abdel-Moneim AE, Elbaz AM, Khidr RE, Badri FB (2020) Effect of in ovo inoculation of Bifidobacterium spp. on growth performance, thyroid activity, ileum histomorphometry, and microbial enumeration of broilers. Probiotics Antimicrob Proteins 12:873–882. https://doi.org/10.1007/s12602-019-09613-x
Wu Y, Lei Z, Wang Y et al (2021) Metabolome and microbiota analysis reveals the conducive effect of Pediococcus acidilactici BCC-1 and xylan oligosaccharides on broiler chickens. Front Microbiol 12:683905. https://doi.org/10.3389/fmicb.2021.683905
Zuo ZH, Shang BJ, Shao YC, Li WY, Sun JS (2019) Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immunol 86:160–168. https://doi.org/10.1016/j.fsi.2018.11.003
Wang T, Teng K, Liu Y et al (2019) Lactobacillus plantarum PFM 105 promotes intestinal development through modulation of gut microbiota in weaning piglets. Front Microbiol 10:90. https://doi.org/10.3389/fmicb.2019.00090
Cheng R, Guo J, Pu F et al (2019) Loading ceftriaxone, vancomycin, and Bifidobacteria bifidum TMC3115 to neonatal mice could differently and consequently affect intestinal microbiota and immunity in adulthood. Sci Rep 9:3254. https://doi.org/10.1038/s41598-018-35737-1
Zou Y, Liang N, Zhang X, Han C, Nan X (2021) Functional differentiation related to decomposing complex carbohydrates of intestinal microbes between two wild zokor species based on 16SrRNA sequences. BMC Vet Res 17:216. https://doi.org/10.1186/s12917-021-02911-z
Dela Cruz PJD, Dagaas CT, Mangubat KMM, Angeles AA, Abanto OD (2019) Dietary effects of commercial probiotics on growth performance, digestibility, and intestinal morphometry of broiler chickens. Trop Anim Health Prod 51:1105–1115. https://doi.org/10.1007/s11250-018-01791-0
Rothhammer V, Mascanfroni ID, Bunse L et al (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22:586–597. https://doi.org/10.1038/nm.4106
Tsvetikova SA, Koshel EI (2020) Microbiota and cancer: host cellular mechanisms activated by gut microbial metabolites. Int J Med Microbiol 310:151425. https://doi.org/10.1016/j.ijmm.2020.151425
Mahdavi M, Laforest-Lapointe I, Masse E (2021) Preventing colorectal cancer through prebiotics. Microorganisms 9:1325. https://doi.org/10.3390/microorganisms9061325
Lee J, d’Aigle J, Atadja L et al (2020) Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res 127:453–465. https://doi.org/10.1161/CIRCRESAHA.119.316448
Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Reichardt N, Duncan SH, Young P et al (2014) Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J 8:1323–1335. https://doi.org/10.1038/ismej.2014.14
Peng Q, Zeng XF, Zhu JL et al (2016) Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult Sci 95:893–900. https://doi.org/10.3382/ps/pev435
Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N (2016) Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 7:185. https://doi.org/10.3389/fmicb.2016.00185
Czerwinski J, Hojberg O, Smulikowska S, Engberg RM, Mieczkowska A (2010) Influence of dietary peas and organic acids and probiotic supplementation on performance and caecal microbial ecology of broiler chickens. Br Poult Sci 51:258–269. https://doi.org/10.1080/00071661003777003
Canfora EE, Jocken JW, Blaak EE (2015) Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11:577–591. https://doi.org/10.1038/nrendo.2015.128
Kim JS, Ingale SL, Kim YW et al (2012) Effect of supplementation of multi-microbe probiotic product on growth performance, apparent digestibility, cecal microbiota and small intestinal morphology of broilers. J Anim Physiol Anim Nutr (Berl) 96:618–626. https://doi.org/10.1111/j.1439-0396.2011.01187.x
Adewole DI, Kim IH, Nyachoti CM (2016) Gut health of pigs: challenge models and response criteria with a critical analysis of the effectiveness of selected feed additives - a review. Asian-Australas J Anim Sci 29:909–924. https://doi.org/10.5713/ajas.15.0795
Xie Y, Liu J, Wang H et al (2020) Effects of fermented feeds and ginseng polysaccharides on the intestinal morphology and microbiota composition of Xuefeng black-bone chicken. PLoS ONE 15:e0237357. https://doi.org/10.1371/journal.pone.0237357
Zahran E, Risha E, Abdelhamid F, Mahgoub HA, Ibrahim T (2014) Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 38:149–157. https://doi.org/10.1016/j.fsi.2014.03.002
Holzapfel W, Arini A, Aeschbacher M et al (2018) Enterococcus faecium SF68 as a model for efficacy and safety evaluation of pharmaceutical probiotics. Benef Microbes 9(3):375–388. https://doi.org/10.3920/BM2017.0148
Duncan SH, Holtrop G, Lobley GE et al (2004) Contribution of acetate to butyrate formation by human faecal bacteria. Br J Nutr 91(6):915–923. https://doi.org/10.1079/BJN20041150
Peng L, Li ZR, Green RS et al (2009) Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 139(9):1619–1625. https://doi.org/10.3945/jn.109.104638
Zimmerman MA, Singh N, Martin PM et al (2012) Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells. Am J Physiol Gastrointest Liver Physiol 302(12):G1405–G1415. https://doi.org/10.1152/ajpgi.00543.2011
Fukuda S, Toh H, Hase K et al (2011) Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 27;469(7331):543–547. https://doi.org/10.1038/nature09646
Rivière A, Selak M, Lantin D et al (2016) Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 7:979. https://doi.org/10.3389/fmicb.2016.00979
Rivière A, Gagnon M, Weckx S et al (2015) Mutual cross-feeding interactions between Bifidobacterium longum subsp. longum NCC2705 and Eubacterium rectale ATCC 33656 explain the bifidogenic and butyrogenic effects of arabinoxylan oligosaccharides. Appl Environ Microbiol 81(22):7767–7781. https://doi.org/10.1128/AEM.02089-15
Li HY, Zhou DD, Gan RY et al (2021) Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: a narrative review. Nutrients 13(9):3211. https://doi.org/10.3390/nu13093211
Funding
This work was financially supported by China Agriculture Research System of MOF and MARA (CARS-41) and the Agricultural Science and Technology Innovation Program (ASTIP-IAS07).
Author information
Authors and Affiliations
Contributions
W.W.: methodology resources, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing. G.D.: methodology resources, formal analysis, data curation, writing—review and editing. W.H.: conceptualization, investigation, data curation. A.L.: supervision, resources. H.Z.: supervision, project administration. S.G.: investigation, resources. T.M.: supervision, project administration, funding acquisition. W.W. and G.D. have an equal contribution to this work. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics
The study was carried out in strict inspection of Experimental Animal Welfare and Ethics Committee of Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (IAS-CAAS, Permit Number: IAS2021-43). All of the tests were carried out in accordance with ethical standards.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, W., Dang, G., Hao, W. et al. Dietary Supplementation of Compound Probiotics Improves Intestinal Health by Modulated Microbiota and Its SCFA Products as Alternatives to In-Feed Antibiotics. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10314-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10314-3