Abstract
Salmonellosis is a disease caused by non-typhoid Salmonella, and although some lactic acid bacteria strains have been shown previously to relieve Salmonellosis symptoms, little has been studied about the preventive mechanism of Lentilactobacillus buchneri (L. buchneri) against Salmonella infection in vivo. Therefore, the L. buchneri was fed to C57BL/6 mice for 10 days to build a protective system of mice to study its prevention and possible mechanisms. The results showed that L. buchneri GX0328-6 alleviated symptoms caused by Salmonella typhimurium infection among C57BL/6 mice, including low survival rate, weight loss, increase in immune organ index and hepatosplenomegaly, and modulated serum immunoglobulin levels and intrinsic immunity. Importantly, the L. buchneri GX0328-6 enhanced the mucosal barrier of the mouse jejunum by upregulating the expression of tight junction proteins such as ZO-1, occludins, and claudins-4 and improved absorptive capacity by increasing the length of mouse jejunal villus and the ratio of villus length to crypt depth and decreasing the crypt depth. L. buchneri GX0328-6 reduced the intestinal proliferation and invasion of Salmonella typhimurium by modulating the expression of antimicrobial peptides in the intestinal tract of mice, and reduced intestinal inflammation and systemic spread in mice by downregulating the expression of IL-6 and promoting the expression of IL-10. Furthermore, L. buchneri GX0328-6 increased the relative abundance of beneficial bacteria colonies and decreased the relative abundance of harmful bacteria in the cecum microflora by modulating the microflora in the cecum contents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last two decades, Salmonella was considered the main pathogen of foodborne diseases worldwide including gastroenteritis [1, 2]. Salmonella stimulates the expression of immune system genes, which involve in the inflammatory responses [3, 4]. Salmonella enterica serovar typhimurium causes non-typhoidal salmonellosis, which is one of the most prevalent foodborne diseases [5]. As an important intracellular bacterium, the infection of Salmonella typhimurium is not only a serious threat to human health but also causes significant economic losses in the agriculture industry [6, 7]. Although salmonellosis can be treated with multiple types of broad-spectrum antibiotics, the limitation includes antibiotic resistance and food safety issues [8,9,10,11]. Therefore, there is an urgent need to find alternative strategies to reduce the transmission of foodborne pathogens in a safer and eco-friendly way [12].
Probiotics have been broadly used in producing food such as cheese, yogurt, and fermented salted fish [13,14,15,16]. The health benefits of fermented food have been confirmed. Probiotics have been described as “organisms and substances that contribute to intestinal microbial homeostasis” [17, 18]. It modulates the immune system, limits pathogen colonization, and controls inflammatory bowel diseases and metabolic disorders [19]. Previous studies found that probiotics alter the mucosal immune system through Toll-like receptor-mediated processes. They promote T helper cell 1 differentiation thereby increasing antibody production, inducing phagocytosis, and enhancing the activities of natural cells. Probiotics can also inhibit nuclear factor light chain enhancers to activate B cell pathways, induce T cell apoptosis, increase anti-inflammatory cytokines such as interleukin (IL)-10, and decrease pro-inflammatory cytokines [19,20,21,22,23,24]. Previous studies also showed that Lactobacillus plantarum P8 could inhibit oocyst shedding as well as improve the general growth performance and intestinal health among broilers infected by Eimeria coccidia [25]. Wang et al. showed that Lactobacillus plantarum could attenuate Clostridium perfringens–induced growth performances and soothe intestinal ecological disorders among broilers by an increased level of short-chain fatty acids therefore improving their intestinal health [26]. Previous studies also showed that taking dietary with Lactobacillus plantarum B1 could increase the number of lactic acid bacteria and the concentration of short-chain fatty acid (SCFA) concentrations in the intestine [27]. Mazkour et al. found that the supplementation with both Bacillus subtilis and Bacillus coagulans improved the growth performance through benefiting intestinal microorganisms in rats [28]. Jang et al. found that the Lactobacillus fermentum improved mice’s immune system by modulating their intestinal flora [29]. Wang et al. represented that Lactobacillus reuteri promoted intestinal development and modulated mucosal immunity in neonatal piglets [30]. In general, different probiotic strains can either promote the growth performance of animals or attenuate the tissue damage caused by foreign pathogens and soothe their intestinal ecological dysregulations.
The Lentilactobacillus buchneri could metabolize lactic acid into acetic acid and 1,2-propanediol under the anaerobic and acidic conditions, therefore maintaining cell activities. In the storage of sugarcane silage, the L. buchneri reduces the loss of dry matter content, maintains its pH value, benefits the lactic acid bacteria (LAB) population, helps the dry matter recovery, and maintains the concentration of WSC, lactic acid, acetic acid, and ethanol concentrations within a healthy range [31,32,33,34]. In general, the application of L. buchneri has strong advantages in feed preservation, particularly in reducing dry matter loss, improving aerobic stability and degradation rate [35, 36]. Additionally, adding L. buchneri also has a lower cost compared to chemical additives [37, 38]. It has also been reported that L. buchneri promotes the growth performance of cattle and regulates the microbial population of silage [31, 35]. However, little is known about its regulation of intestinal mucosal barriers such as microflora in monogastric animals, and neither does the mechanism of L. buchneri in the prevention of Salmonella typhimurium infection, nor its probiotic potential has been well studied. Therefore, this study tends to investigate the preventive and protective effects of L. buchneri in improving the function of intestinal mucosal barriers and its immune functions against Salmonella typhimurium infection among C57/BL6 mice.
Results
Effects of L. buchneri GX0328-6 on Immune Organs, Body Weights, and Survival Rates
Figure 1a shows that oral administration of L. buchneri GX0328-6 affects body weight changes in Salmonella typhimurium–infected mice. On day 5 after infection, body weight significantly decreased among mice in the positive group (SM022) (P < 0.05). Pretreatment with L. buchneri GX0328-6 reduced the weight loss caused by the infection of Salmonella typhimurium (P = 0.059). Figure 1b shows that the oral administration of L. buchneri GX0328-6 could improve the survival rate of Salmonella typhimurium–infected mice. The survival rate was 100% in both the negative control (CON) and L. buchneri-treated groups, 91.67% in the prophylactic group (LB + SM022) while only 50% of the positive group infected with Salmonella typhimurium survived. In addition, L. buchneri GX0328-6 pretreatment reduced the thymic and splenic indices in mice, especially the thymic index (P < 0.05) (Fig. 1c).
The body weight change (a), survival rate (b), and immune organ index (c) in mice treated or not during 10 days by L. buchneri GX0328-6 and then infected or not with Salmonella typhimurium SM022. The negative control group (CON) and L. buchneri group (LB) were only fed with saline and L. buchneri GX0328-6 respectively for 10 consecutive days; the prophylactic group (LB + SM022) and the positive group (SM022) were given L. buchneri GX0328-6 and saline respectively for 10 consecutive days, and then the mice were fed with Salmonella typhimurium SM022. *P < 0.05, ns had no statistical significance. (one-way ANOVA)
The probiotic L. buchneri GX0328-6 Reduces Transmission of Salmonella Typhimurium Among C57BL/6 Mice
Compared to the negative control group, we found that the IgG level was significantly increased (P < 0.05) among Salmonella typhimurium–infected mice. Meanwhile, the levels of IgG, IgA, and IgM in mice infected with Salmonella typhimurium after pretreatment with L. buchneri GX0328-6 had no significant difference compared with the positive group. However, when treated with L. buchneri GX0328-6 only, mice showed a significant increase in IgG (P < 0.01) while the level of both IgA and IgM were significantly decreased (P < 0.05) (Fig. 2a, b, and c). Interestingly, among L. buchneri GX0328-6 pretreated mice, we found that pathogen translocation was significantly reduced in the spleen, the liver, and the cecum compared to the positive group; particularly, the largest amount of reduction was observed in their cecum (Fig. 2d, e, f).
The levels of immunoglobulin IgG (a), IgM (b), and IgA (c) in mice treated or not during 10 days by L. buchneri GX0328-6 and then infected or not with Salmonella typhimurium SM022, the contents of Salmonella typhimurium in mouse liver (d), spleen (e) and cecum (f) tissues in mice treated or not during 10 days by L. buchneri GX0328-6 and then infected with Salmonella typhimurium SM022 (n = 10) *P < 0.05, **P < 0.01, ****P < 0.0001, ns had no statistical significance (one-way ANOVA)
Pretreating with L. buchneri GX0328-6 Could Reduce the Severity of Salmonella Typhimurium Infection Among C57BL/6 Mice
Compared with the prophylactic group, the liver and spleen of positive controls were significantly enlarged, and the color of these livers became lighter (Appendix 1a). In addition, reduced food intake occurs among mice in the positive group with reduced movements and self-grooming, as well as disheveled hair that lost its luster (Appendix 1b). We also investigated the morphological changes of the liver under infection by evaluating the frequency and severity of inflammatory foci. We observed the decrease of inflammatory cell number and hepatocyte necrosis in the liver of mice in the prophylactic group compared to the positive group (Fig. 3). In addition, small intestinal villi and the ratio of small intestinal villi to crypt depth were significantly increased, and crypt depth was significantly decreased in the prophylactic group compared to the positive group (Fig. 4).
The representative liver micrograph of mice treated or not during 10 days by L. buchneri GX0328-6 and then infected with Salmonella typhimurium SM022. The black thin arrow indicates that there are inflammatory cells (10 ×); the red thick arrow indicates necrosis of liver cells (40 ×); the black triangle box indicates the aggregation of red blood cells (40 ×). b is the result of liver inflammation–related cell frequency. ***P < 0.001 (one-way ANOVA)
The pathological changes (a) of jejunum mucosa in mice, the villus length (b), the crypt depth (c) and the ratio (d) of villus and crypt depth in the jejunum of mice treated or not during 10 days by L. buchneri GX0328-6 and then infected with Salmonella typhimurium SM022. **P < 0.01; ***P < 0.001 (one-way ANOVA)
L. buchneri GX0328-6 Regulates mRNA Expression of Inflammatory Cytokines, Antimicrobial Peptides, and Intestinal Mucosa–Associated Proteins During Salmonella Typhimurium C57BL/6 Infection
Figure 5a shows that the mRNA expression of the cytokine IL-6 with potential pro-inflammatory function was significantly lower in the jejunum of the prophylactic group compared to the group of positive controls (P < 0.005). Meanwhile, there was a trend of decreasing but not yet statistically significant mRNA expression for the anti-inflammatory cytokine IL-10 (Fig. 5b). On the other hand, both groups treated by the prophylactic group and the L. buchneri group showed an increase of the Ang4 mRNA expression, while the mRNA expression of REG III decreased. Our data showed that Salmonella typhimurium significantly upregulated the expression of REG III mRNA, while it downregulated in the prophylactic group (Fig. 5c, d). In addition, our data represented that Salmonella typhimurium decreased the expression of jejunal ZO-1, occludins, and claudins-4, but the mRNA expression of all three was significantly increased (P < 0.05) when pretreated the mice with L. buchneri GX0328-6 before infection with Salmonella typhimurium (Fig. 6).
mRNA relative expression amounts of inflammation-related cytokines IL-6 (a) and IL-10 (b) and antibacterial peptides Ang4 (c) and REGIII (d) in the jejunum of mice treated or not during 10 days by L. buchneri GX0328-6 and then infected or not with Salmonella typhimurium SM022. (n = 6) *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA)
L. buchneri GX0328-6 Could Regulate Intestinal Microflora in Mice
Using Illumina MiSeq 16S amplification and sequencing technique, we investigated the effects which L. buchneri GX0328-6 makes on modifying the population of cecum microbiota. As shown in Figs. 7 and 8, at the phylum level, compared to the negative control group, the relative proportion of Bacteroidota (54.78%) decreased in positive controls, while the relative proportion of their Firmicutes (34.48%) increased. Compared to the negative control group, the proportion of Bacteroidota (54.78%) in the positive group decreased, Firmicutes (34.48%) increased, and Campylobacter (4.66%), Proteobacteria (1.98%), and Spirochaeta (1.53%) did not show significant changes, while the prevention group not only reduced the increase in Firmicutes (25.45%) caused by Salmonella typhimurium SM022, but also significantly reduced the proportion of Campylobacter (1.99%) (P < 0.05) and Proteobacteria (1.09%) (P = 0.0925) (Fig. 9a, b). The relative proportion of the harmful bacteria, Spirochaetota (0.49%), was also reduced, although this change was not statistically significant. In addition, the relative proportion of Bacteroidota (68.16%) was also increased. Compared to the negative control group, the Muribiculaceae (32.94%) decreased in the positive control group, while the relative proportion of Muribaculum (3.75%), Prevotellaceae_UCG-001 (2.59%), Lachnocolstridium (7.64%), and Ruminococcus (0.68%) all increased. No significant changes were observed for the Helicobacter (4.65%). Additionally, compared with the positive control group, the L. buchneri-pretreated group (LB_SM022), an increased population of the Lachnospiraceae_NK4A136_group (12.65%) was observed together with a significant increase of the Muribiculaceae (56.71%) (P < 0.05) (data represented in Fig. 9c). Furthermore, it also inhibited the increasing of Muribaculum (1.93%), Prevotellaceae_UCG-001 (2.04%), Bacteroides (1.22%), Lachnocolstridium (0.28%), and Helicobacter (1.98%) after the infection of Salmonella typhimurium SM022. Particularly, it significantly inhibited the population expenditure of Helicobacter (1.98%) (P < 0.05) (Fig. 9d).
Materials and Methods
The Preparation of Bacterial Strains
The testing strain, Salmonella typhimurium SM022, is a mutant strain of the wild-type Salmonella typhimurium ATCC 14028s [39]. The concentration of 1×108CFU/mL of Salmonella typhimurium SM022 we choose for subsequent experiments depended on our preliminary experiment on the median lethal dose of Salmonella typhimurium SM022. The Lentilactobacillus buchneri (L. buchneri) was isolated from the traditional Chinese fermented plant food sauerkraut collected in Guangxi and named Lentilactobacillus buchneri GXNN20210328-6 (L. buchneri GX0328-6). The sequence of L. buchneri GX0328-6 was uploaded to NCBI by 16S rRNA comparison with the registration number MZ461959.1. According to the World Gastroenterology Organization Global Guidelines on Probiotics and Prebiotics [40] and the International Scientific Association for Probiotics and Prebiotics (ISAPP) expert team, Binda et al. [41] put forward in the probiotic application guide that the recommended amount of probiotics is 108~1011CFU/day, while previous studies have reported that Lactobacillus alleviated Salmonella typhimurium infection in mice at a concentration of 108CFU/mL [42, 43], and the concentration of 108 CFU for L. buchneri GX0328-6 was selected in the present study. Salmonella typhimurium SM022 and L. buchneri GX0328-6 were grown to log phase in LB broth and MRS broth (Beijing Landbridge Technology Co., Ltd.), respectively. Bacterial cells were collected by centrifugation at 4000 rpm for 10 min, rinsed twice with saline (NS), and then adjusted the concentration of both bacterial suspensions to 108 CFU/ mL before feeding to mice.
Animal Research
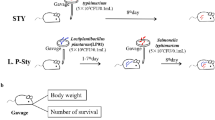
In this study, eighty-eight 4-week-old C57BL/6 mice were housed at 22–26 °C, 20% humidity with a light/dark cycle for 12 h under standardized conditions. All animals were arbitrary feed of standard diet and distilled water. After 1 week of acclimation, mice were randomly divided into 4 groups (n = 22). In the negative control group (CON): each mouse was fed with 0.2 mL of saline alone with the standard diet for 10 days; in the L. buchneri group (LB): each mouse was fed with 0.2 mL of 108 CFU/mL of L. buchneri GX0328-6 solution for 10 days; in the prophylactic group (LB + SM022): each mouse was fed with 0.2 mL 108 CFU/ mL L. buchneri GX0328-6 for 10 days, and then each mouse was treated by 0.2 mL 108 CFU/mL Salmonella typhimurium SM022 for 1 day; in the positive group (SM022): each mouse was fed with 0.2 mL saline for 10 days, and then each mouse was treated by 0.2 mL 108 CFU/mL Salmonella typhimurium SM022 for 1 day. On the 5th day after the Salmonella typhimurium SM022 infection, 10 mice from each group were euthanized, and tissue was collected for further analysis, while other mice were used for testing the survival rate.
The Measurement of the Immune Organ Index and Survival Rate
Each mouse was weighed and euthanized, and the thymus and spleen were collected/weighed respectively after carefully removing surrounding tissue and fat. The weight of each organ was recorded, and the immune organ index was calculated based on the following formula: immune organ index (%) = immune organ weight/mouse body weight × 100%. The weight of each mouse before and after infection with Salmonella typhimurium was observed, and the survival curves were plotted based on the observational data.
The Determination of Salmonella Translocations
The liver, spleen, and cecum samples from each mouse were isolated, weighed, and homogenized in sterile phosphate–buffered saline (PBS, 1:10, w/v). For each organ, the supernatant of homogenized solution was further diluted into a series of decimal dilutions, incubating at 37 °C for 24 h, and then inoculating on bismuth subsulfite agar medium (Guangdong Huan Kai Microbiology Technology Co., Ltd., Guangdong, China) for Salmonella-specific enumeration. The expression threshold of Salmonella typhimurium translocation was log10 CFU/g of each organ sample.
The Immunoglobulin and Cytokine Analysis
For serum samples, the level of immunoglobulin IgG, IgM, and IgA were quantified by using a mouse-specific ELISA kit (Jiangsu Jingmei Biotechnology Co., Ltd., Jiangsu, China) according to the manufacturer’s instructions. The OD (optical density) value of each sample was measured by a Thermo Scientific microplate reader at 450 nm wavelength.
The Relative Expression of Cytokines in Jejunum Samples
A small section (approximately 1 cm) of the jejunum sample was collected and stored at − 80 °C in a 1.5-mL RNAse-free EP tube until further analysis. RNA was extracted according to the RNA pure Tissue&Cell Kit (DNase I) (Jiangsu Kangwei Biotechnology Co., Ltd., Jiangsu, China) procedure. The cDNA of each sample was produced by using the HiFi Script cDNA Synthesis Kit (Kangwei Biotechnology Co., Ltd., Jiangsu, China) according to its manual instructions. Quantitative PCR (qPCR) was performed using QuantiTect SYBR Green PCR Kit (Kangwei Biotechnology Co., Ltd., Jiangsu, China). Primer sequences for each amplification are summarized in Table 1. Amplification reactions were performed by using 10 μl of SYBR green master mix, 50 ng of cDNA, and distilled water to make a total volume of 20 μl. For each gene, the expression level of the control group which feed saline was used as inner control. All results were plotted in a bar graph based on folders of changes for the expression of each target gene. The mean and standard deviation (2−ΔΔCT) of the cytokine expression level was calculated following the previously published protocol [43].
The Histological Analysis
For each mouse, the jejunal intestinal segment was collected, briefly rinsed with PBS, and fixed in buffered 4% formaldehyde-PBS solution followed by conventional paraffin embedding. For liver samples, at least two Sects. (4–5 μm/slice) were prepared from each sample, and either hematoxylin or eosin (HE) staining was applied. Histological results were observed by light microscopy (Nikon model eclipse E200 type) and analyzed by a pathologist who was blind to the experimental condition of each sample. To determine villi length and crypt depth, at least 8 villi and crypt foci were measured from different observational fields of each respective tissue section. All images were quantified by using the Image View software (Shanghai Puch Optoelectronics Technology Co., Ltd., Shanghai, China).
The Analysis of Gut Microbiota
The gut microbiota was observed by using the protocol previously described [44]. Briefly, the V3-V4 region of the 16S rRNA was amplified from the total genomic DNA of cecum contents by using barcoded primers 343F and 798R. PCR amplification products (amplicon) were purified by using Agencourt AMPure XP beads (Beckman Coulter Co., USA) and quantified by the Qubit dsDNA Assay. The 16S rRNA gene amplification products were sequenced and analyzed by Oei Biotech (Shanghai, China). Sequencing data were processed by using the Microbial Ecology 2 (QIIME2) pipeline for quantitative analysis. Sequences were clustered into operational taxonomic units (OTUs) by 97% identity level using the Green genes database (ver. 13_5). Microbial diversity in cecum contents was estimated using alpha diversity including Chao1 index [45] and Shannon index [46] to determine significant differences between groups. Furthermore, in addition, unobserved data were reconstructed based on data from the KEGG pathway database (Genome Net; https://www.genome.jp/kegg/path way.html) to identify functional genes in the microbial communities of the samples [47].
The Statistical Analysis
The statistical analysis was evaluated by the two-way ANOVA or one-way ANOVA, followed by a post hoc Tukey’s test, with SPSS Statistics version 25.0. The immune organ index of mice was analyzed by two-way ANOVA, and other experimental results were analyzed by one-way ANOVA. The gut microbiota of mice was statistically analyzed using Mann–Whitney U Test, with no error detection rate (FDR) correction. Only results with P < 0.05 were considered statistically significant.
Discussion
It has been reported that probiotics could maintain the barrier function of intestinal epithelial cells in mammal model [48,49,50]. Feeding probiotics inhibited the growth of Escherichia coli in the jejunum, colon, and ileum [51]. The L. buchneri GX0328-6 strain showed good probiotic properties, which was isolated from sauerkraut, a plant-sourced, traditional fermented food collected from Guangxi province, China. Compared to the positive control group, we observed the gain of average body weight among mice that were pretreated with L. buchneri GX0328-6 before being infected by Salmonella typhimurium (Fig. 1a). Previous studies have shown that the protection function of probiotic treatment includes the prevention of weight loss under the infection of Salmonella among animals [52]. This conclusion is consistent with the results of the present study. Figure 1b shows that feeding the L. buchneri GX0328-6 as a pretreatment before the Salmonella typhimurium infection could significantly increase the survival rate from 50% (mice without GX0328-6 treatment) to 91.67%. Additionally, the L. buchneri GX0328-6 treatment also decreased the symptoms of the Salmonella infection as well as a healthier look of their coat and skin appearance (Appendix 1) and significantly reduced thymic index (P < 0.05) (Fig. 1c). This suggests that L. buchneri GX0328-6 can effectively alleviate clinical symptoms and mortality in mice during Salmonella typhimurium infection. Zhang et al. [53] showed that feeding L. buchneri in chicks before the Salmonella infection could significantly reduce dysentery and improve survival rate, which is consistent with the results in mice after the treatment of L. buchneri GX0328-6 in this study. In contrast, Santos et al. found that Lactobacillus plantarum 286 failed to protect conventional mice from Salmonella typhimurium infection, and there was no statistically significant difference among groups, nor was the survival rate changed [54]. This indicates that not all probiotic strains could improve the survival rate of Salmonella-infected animals. It also reaffirms the potential of the L. buchneri GX0328-6 as a probiotic in protecting mice from the poor outcome of the Salmonella typhimurium infection.
Studies have shown that strong antigen-specific cellular and humoral immune responses are associated with defenses against the Salmonella typhimurium infection [55]. Oxidative outbreaks of invasive Salmonella typhimurium strains were positively correlated with serum levels of antibodies such as immunoglobulins IgG and IgM [56]. Despite the importance of antibodies in the control of Salmonella infection [57], little work has been done on analyzing the regulation of serum antibodies after animals were treated by the L. buchneri GX0328-6. This ignited our interest in revealing the role of serum antibodies against Salmonella typhimurium. Our data showed (Fig. 2a, b, c) that mice infected with the Salmonella typhimurium had significantly increased levels of IgG (P < 0.05) and no significant differences in levels of IgM and IgA (P > 0.05) compared to controls. Other studies have also found elevated IgG antibody levels during 1–4 weeks after infection with Salmonella typhimurium inoculation [58]. Interestingly, feeding L. buchneri GX0328-6 alone to mice increased immunoglobulin IgG antibody levels, but IgG expression in mice infected with Salmonella typhimurium after pretreatment with L. buchneri GX0328-6 was not significantly different from that in mice infected with Salmonella typhimurium only. It is possible that L. buchneri-induced immunoglobulins form an antigen–antibody binding reaction with Salmonella typhimurium, thereby slowing the rise in IgG antibody levels. However, the exact mechanism requires further studies before it can be resolved experimentally.
In the present study, the number of Salmonella typhimurium detections was significantly lower in all organs of the prophylactic group pretreated with L. buchneri GX0328-6 compared to the positive group (Fig. 2e, f, g). After oral administration of Salmonella typhimurium, Salmonella typhimurium entered the intestine of mice and adhered to intestinal epithelial cells, thereby promoting bacterial colonization in the intestine through its type I hairs [59]. With the migration of phagocytes from the mesenteric lymph nodes, it eventually spreads throughout the body and invades different organs or tissues such as the liver and spleen, causing various clinical symptoms [60]. Invasion of Salmonella typhimurium leads to hepatosplenomegaly and eventually organ failure [61]. In the present study, L. buchneri GX0328-6 reduced the load of Salmonella typhimurium in these organs and alleviated Salmonella typhimurium–induced hepatosplenomegaly (Supplementary Figure) Similar results were observed by Acurcio et al. [43] after feeding BALB/c mice with milk fermented by Lactobacillus paracasei NCC 2461 (ST11). In addition, H&E staining results showed that L. buchneri GX0328-6 pretreatment significantly reduced liver frequency, hepatocyte damage, and inflammatory cells (Fig. 3). These data suggest that pre-feeding of L. buchneri GX0328-6 has a positive effect on inhibiting the proliferation and invasion of Salmonella typhimurium in the intestinal tract of mice and has a protective effect on organ damage in mice. Studies have shown that the use of certain probiotics can improve the function of the small intestinal barrier by modulating the immune response, thus blocking the translocation of pathogenic bacteria to sterile organs such as the liver [62]. As a result, the survival, proliferation, and spread of pathogens in visceral organs are controlled. The ability of L. buchneri GX0328-6 to reduce liver tissue damage may be related to these mechanisms. Interestingly, studies have shown that the spread of Salmonella to the liver and spleen is also associated with increased permeability of the intestinal barrier [60]. In contrast, the mechanism of action of L. buchneri in regulating intestinal barrier function in animals is not known. Therefore, we next looked at the preventive protective effect of L. buchneri by measuring the intestinal mucosal barrier. The intestinal mucosal barrier consists of four parts: physical barrier, immune barrier, chemical barrier, and biological barrier, which can effectively protect against the invasion of foreign pathogenic microorganisms.
Our results showed that Salmonella typhimurium significantly decreased villi length and villi length to crypt depth ratio and increased jejunal crypt depth in mice. This is consistent with previous findings [63, 64]. In contrast, L. buchneri GX0328-6 pretreatment significantly increased mouse jejunal villus height and the ratio of intestinal villus height to crypt depth and significantly decreased mouse jejunal crypt depth (Fig. 4b, c, d). Prevention by L. buchneri GX0328-6 effectively counteracted the effects of Salmonella typhimurium SM022 on jejunal villus height and the ratio of intestinal villus height to crypt depth in normal mice. It has been shown that the addition of the probiotic Enterococcus faecalis to the diet increased jejunal villus height and ileal villus height [65]. In addition, the addition of a variant of Bacillus subtilis to the diet resulted in the same changes [66]. The same results were obtained in the present study. Also, our pathological histological analysis showed that L. buchneri GX0328-6 was effective in protecting the integrity of the small intestinal mucosal tissue and that the intestinal tissues were not damaged (Fig. 4a). In addition, prevention by L. buchneri GX0328-6 effectively counteracted the effects of Salmonella typhimurium SM022 on jejunal villus height and the ratio of intestinal villus height to crypt depth in normal mice. Taken together, these results suggest that L. buchneri GX0328-6 protects the integrity of intestinal epithelial tissue. Furthermore, the length of the villi was significantly and positively correlated with the number of villi epithelial cells, and only mature villi cells had the function of nutrient absorption. Therefore, when the villi are long, there are more mature cells, and the nutrient absorption capacity is strong [67]. The crypt depth reflects the generation rate of intestinal epithelial cells, and a shallower crypt indicates an increase in the maturation rate of intestinal epithelial cells and enhanced secretion function. The ratio of villi length to crypt depth (V/C ratio) was used to reflect the functional status of the small intestine, and when the V/C value increased, it indicated that the villi had an increased ability to absorb nutrients, and vice versa [68, 69]. These results also suggest that L. buchneri GX0328-6 enhances intestinal absorption in mice.
In this study, the expression of the tight junction protein gene (ZO-1), occludins, and claudins-4 was upregulated in the intestine of mice in both the L. buchneri group and the prophylactic group (Fig. 6a, b, c). The expression of the tight junction proteins ZO-1 and occludins was reported to be reduced after Salmonella typhimurium infection [70]. Similar results were obtained from our experiments. Interestingly, we also found that Salmonella typhimurium also reduced the expression of claudins-4. Proteins such as ZOs, occludins, and claudins are tight junction structures in the intestine [71]. The intestinal epithelial barrier is the main site of nutrient absorption in the intestine and the first barrier for animals to resist pathogenic microorganisms and protect the organism from invasion by pathogenic microorganisms, and the intestinal tight junctions are the most critical part of the intestinal epithelium [72]. The ability of L. buchneri GX0328-6 to significantly increase these genes in this study may be a result of L. buchneri GX0328-6 expressing AvrA, as AvrA has been reported to be an effector in stabilizing intestinal tight junctions such as ZO-1 and occludins [73]. Previous studies have reported that certain probiotics significantly maintain intestinal epithelial barrier function in mammals [48,49,50]. The experimental results of the present study also supported this idea. These results suggest that L. buchneri GX0328-6 can protect the mouse intestine from Salmonella typhimurium by increasing the length of intestinal villi, decreasing the depth of intestinal crypts, and increasing the tightly connected structures of the intestine as physical barriers.
Salmonella typhimurium induces intense intestinal inflammation in several agricultural animal hosts such as cattle, pigs, and poultry [74,75,76,77]. During the inflammatory response of the intestine, the intestinal mucosal barrier is damaged, leading to the recruitment of immune cells at the site of inflammation [78]. Some probiotics have been reported to reduce the severity of experimental colitis and improve intestinal inflammation in mice [79]. However, little has been reported so far on the inflammation-attenuating effect of the probiotic L. buchneri in vivo. Studies have shown that feeding probiotics significantly increased IL-6 and IL-10 levels in broiler liver [80] and reduced the expression of inflammatory factors in weaned piglets [81], which had a modulating effect on the proliferation and differentiation of immune system cells. While there are relatively few applications and studies on L. buchneri in the intestine to regulate IL-6 and other intestinal mucosal immunity, therefore, in this study, we measured IL-6 and IL-10 contents in the jejunum of C57BL/6 mice to investigate the effect of L. buchneri GX0328-6 on intestinal mucosal immunity in mice. Our data showed that the gene expression of IL-6 was significantly reduced in the jejunum of mice in both the prophylactic and L. buchneri groups compared to the positive group. In contrast, administration of L. buchneri GX0328-6 alone significantly increased IL-10 gene expression, although IL-10 was also increased in mice pretreated with L. buchneri GX0328-6 and then infected with Salmonella typhimurium (Fig. 5a, b). This finding is consistent with the development of probiotics for the treatment of intestinal inflammation [67,68,69]. IL-6 is commonly considered a pro-inflammatory cytokine produced by classically activated macrophage 1, which has a pro-inflammatory effect on chronic inflammation and autoimmunity [82]. IL-10, on the other hand, is produced by selectively activated macrophage 2 and is usually considered an anti-inflammatory cytokine [83]. IL-10 is closely associated with the prevention of mucosal inflammation by acting on Treg cells or macrophages to prevent inflammatory responses [84]. The results of this study suggest that L. buchneri GX0328-6 ameliorates intestinal inflammation by modulating inflammation-associated cytokines and thereby enhancing intestinal immune barrier function.
In addition to the above immune and physical barriers, the intestinal mucosal barrier also has a chemical and microbial barrier. The mucus layer of the chemical barrier is an important line of defense to prevent pathogenic microorganisms from directly contacting intestinal epithelial cells, and in combination with antimicrobial substances secreted into the mucus layer such as antimicrobial peptides (AMPs) including defensins, substances such as lysozyme and endogenous antimicrobial peptide-like substances defensins and regenerating islet derived protein 3 (REGIII), among others, constitute a chemical barrier [85, 86]. Antimicrobial REG III proteins play an important role in maintaining intestinal homeostasis by spatially separating bacteria, preventing potentially harmful immune responses, and protecting the host from infection [87,88,89]. In addition, it has been shown that Ang4 may have antimicrobial effects [89]. Therefore, we determined the expression of the antimicrobial peptide Ang4 and REG III by qPCR assay to investigate the effect of L. buchneri GX0328-6 on the intestinal mucosal barrier in mice infected with Salmonella typhimurium. In this study, infection with Salmonella typhimurium significantly upregulated the mRNA expression of REG III, while pretreatment with L. buchneri GX0328-6 followed by infection with Salmonella typhimurium significantly decreased the mRNA expression of REG III and also significantly increased the mRNA expression of Ang4. In addition, administration of L. buchneri alone also significantly increased the mRNA expression of Ang4 (Fig. 5c, d). Our results suggest that L. buchneri GX0328-6 can promote the intestinal secretion of the antimicrobial substance Ang4 to defend against Salmonella typhimurium invasion. These results are consistent with the translocation results we observed for Salmonella typhimurium. L. buchneri has been shown to significantly induce the expression of REG III at the ileal crypt, ileal villi, and colon after intestinal colonization [90]. However, the results of the present study were contrary to this. Our results found that pretreatment with L. buchneri GX0328-6 followed by reinfection significantly downregulated REG III expression; instead, Salmonella typhimurium resulted in upregulation of REG III expression. It is possible that REG III, an important repressor in the natural immune system, is abundantly expressed after intestinal damage and enhances natural immune defense in the early stages of inflammation [89]. One study has shown that REG III protein is also consistently increased with increasing levels of inflammation [91]. This also suggests to us that the abnormally high REG III did not suppress the amount of Salmonella typhimurium colonization in the intestine of mice well; instead, the intestinal tissues were further disrupted. Therefore, when there is a strong inflammatory response in the mouse intestine, REG III may be regulated by these inflammatory factors and promote abnormal REG III expression with the increase of inflammation. And at this time, the intestinal immune system is severely disrupted only by the massive expression of REG III can no longer inhibit the invading microorganisms; instead, pro-inflammatory cytokines may interact with REG III to play an important role in the amplification of inflammation, but the specific mechanism still needs further study. In addition, L. buchneri GX0328-6 pre-treatment followed by reinfection significantly downregulated REG III expression. This suggests that L. buchneri GX0328-6 alleviated the intestinal inflammatory pathway likely by affecting inflammation-associated cytokines and thus reducing the abnormal expression of REG III. However, the specific regulatory pathways and mechanisms of L. buchneri GX0328-6 on related cytokines and REG III need to be further investigated.
The microbial barrier consists of a large and diverse community of microorganisms located in the intestinal lumen [92]. Currently, gut microbes have become an integral part of human health research [93]. Gut microbes form a symbiotic ecosystem, which maintains the homeostatic balance in humans and animals. However, this balance may be disrupted by pathological conditions that interfere with intestinal physiology [94]. Studies have shown that probiotics not only improve the intestinal tract and promote the reconstruction of the intestinal mucosa [95] but also improve the intestinal microecology by producing different metabolites that inhibit the growth of pathogenic bacteria and promote the growth of beneficial bacteria [96, 97]. For example, strains of Lactobacillus paracasei can be used to prevent Salmonella typhimurium infections [43]. It has been shown that the diversity and abundance of bacterial species is an aspect of a healthy intestinal microbiota [98]. To investigate whether L. buchneri GX0328-6 could maintain the microbial barrier in the intestine by modulating microorganisms, we determined the intestinal flora characteristics of mice in the control, prophylactic, and positive groups. In the present study, Salmonella typhimurium infection of C57BL/6 mice was able to reduce the abundance of intestinal Bacteroidetes. while L. buchneri GX0328-6 not only enhanced the population of Bacteroidetes to some extent but also inhibited the colonization of the intestine by certain pathogens (Figs. 7, 9). Bacteroidetes is a beneficial bacterium, one of the two most abundant phyla in the intestinal microbiome, that directly mediates the metabolism of carbohydrates, steroids, bile acids, and sugars [99, 100]. Furthermore, our results suggest that Salmonella typhimurium infection of C57BL/6 mice altered the structure of the mouse intestinal microbial community by increasing the relative abundance of pathogens of Protebacterota and Campilobacterota. In contrast, early administration of L. buchneri GX0328-6 reduced the proportion of harmful bacteria such as Protebacterota, Campilobacterota, and Helicobacter (Figs. 7, 9). Alterations in intestinal morphology, function, and bacterial flora have been reported to be caused by inflammation. Pathogenic intestinal flora may negatively affect the nutrition of patients and their metabolic efficiency by decreasing microbiota diversity, thus reducing the production of beneficial metabolites [101]. Salmonella infection leads to an increase in the number of potential pathogens and parthenogenic anaerobes in the cecum microbiota directly disrupting the intestinal flora ecosystem, which leads to malnutrition of the intestinal flora and causes intestinal inflammation [102, 103]. After feeding L. buchneri GX0328-6, the contents of the mouse cecum showed an increase in the dominant flora, such as the Bacteroidetes, and a decrease in the harmful flora, such as Campilobacterota, with changes in their colony structure, which may be due to the fact that L. buchneri GX0328-6 promotes the colonization of beneficial flora, decreases the number of harmful bacteria, and attenuates the inflammatory response caused by harmful bacteria through the production of different metabolites. These results are consistent with the results observed in the immune barrier in which the pro-inflammatory cytokine IL-6 was reduced, and the anti-inflammatory cytokine IL-10 was elevated in the intestinal mucosal tissue. Thus, L. buchneri GX0328-6 prophylaxis can effectively reduce the disruption of intestinal flora structure.
Our results found that Salmonella typhimurium infection of C57BL/6 mice increased the abundance of Lachnocolstridium, Muribaculum, Prevotellaceae_UCG-001, and Ruminococcus. In contrast, feeding L. buchneri GX0328-6 reduced the abundance of these flora and Bacteroides (Fig. 8). Lachnocolstridium, which constitutes an important component of the intestinal flora, can exert anti-inflammatory effects and plays a role in homeostasis in vivo [104]. Prevotellaceae has been shown to increase the severity of DSS-induced colitis in mice [105]. And Prevotella and Bacteroides are the main species in the healthy human intestinal flora that produce acetate and propionate from complex carbohydrates to provide nutrition and maintain the normal physiological functions of the intestine [106]. Salmonella typhimurium infection of C57BL/6 mice increased the abundance of Lachnocolstridium, Muribaculum, Prevotellaceae_UCG-001, and Ruminococcus. It may be that the organism’s microflora is resistant to foreign invasive pathogens. In addition, compared to the positive group, the prevention group showed an increased abundance of Muribiculaceae and Lachnospiraceae_NK4A136_group, Muribiculaceae formerly known as S24-7 family, which is the main bacterial group in the mouse intestine [107, 108]. The relative abundance of Muribaculaceae was negatively correlated with pro-inflammatory cytokines and positively correlated with the expression levels of tight junction protein and mucin 2 [109]. The Lachnospiraceae_NK4A136_group is a representative butyrate-producing bacterium that maintains intestinal barrier integrity in mice and is negatively correlated with intestinal permeability. Butyrate, one of the major SCFAs produced by the microbiota, is important in maintaining gastrointestinal health due to its ability to enhance epithelial barrier integrity and inhibit inflammation [110]. Therefore, Muribiculaceae and Lachnospiraceae_NK4A136_group are important in maintaining the normal state of the intestine in mice [111]. It also indicates that L. buchneri GX0328-6 can prevent damage to intestinal tissues by Salmonella typhimurium by increasing the abundance of beneficial bacteria in the intestinal flora and by inhibiting certain pathogenic bacteria.
Conclusion
This study showed that the L. buchneri GX0328-6 attenuated the symptoms caused by the Salmonella typhimurium-infection among C57BL/6 mice, significantly increased their survival rate, and had a significant preventive protection effect. The possible mechanism is through the ability of the L. buchneri GX0328-6 to modulate the level of serum immunoglobulins, enhance the intestinal mucosal barrier, and reduce the effect of the Salmonella typhimurium on the intestinal invasion of mice. Immunoglobulin levels did not change in mice after infection with Salmonella typhimurium probably because L. buchneri-induced immunoglobulins formed an antigen–antibody binding reaction with the Salmonella typhimurium during the infection cycle. Nevertheless, pre-treated with L. buchneri GX0328-6 followed by the Salmonella typhimurium infection also modulated the intrinsic immunity of mice. The L. buchneri enhanced the mucosal barrier and absorptive capacity of mouse jejunum by upregulating the expression levels of tight junctions such as ZO-1, occludins, and claudins-4 and increasing the ratio of villi length and villi length to crypt depth in mouse jejunum and decreasing the crypt depth through GX0328-6. Interestingly, the L. buchneri GX0328-6 reduced intestinal proliferation and invasion of Salmonella typhimurium by promoting the expression of antimicrobial peptides in the mouse intestine, and reduced intestinal inflammation and systemic spread in mice by downregulating the expression of pro-inflammatory cytokine IL-6 and promoting the expression of anti-inflammatory cytokine IL-10 in the jejunum. In addition, the L. buchneri GX0328-6 increased the relative abundance of beneficial bacteria and decreased the relative abundance of harmful bacteria in the cecum microflora by modulating the microflora in the cecum contents.
Availability of Data and Materials
The data and materials presented in this study are available on request from the corresponding author.
Code Availability
Not applicable.
References
Fantasia M, Filetici E (1994) Salmonella enteritidis in Italy. Int J Food Microbiol 21(1–2):7–13. https://doi.org/10.1016/0168-1605(94)90194-5
Omwandho C, Kubota T (2010) Salmonella enterica serovar Enteritidis: a mini-review of contamination routes and limitations to effective control. Japan Agricultural Research Quarterly 44(1):7–16
Crhanova M, Hradecka H, Faldynova M, Matulova M, Havlickova H, Sisak F et al (2011) Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect Immun 79(7):2755–2763. https://doi.org/10.1128/IAI.01375-10
Giansanti F, Giardi MF, Botti D (2006) Avian cytokines–an overview. Curr Pharm Des 12(24):3083–3099. https://doi.org/10.2174/138161206777947542
Won G, Lee JH (2017) Salmonella Typhimurium, the major causative agent of foodborne illness inactivated by a phage lysis system provides effective protection against lethal challenge by induction of robust cell-mediated immune responses and activation of dendritic cells. Vet Res 48(1):66. https://doi.org/10.1186/s13567-017-0474-x
Herrero-Fresno A, Olsen JE (2018) Salmonella typhimurium metabolism affects virulence in the host - a mini-review. Food Microbiol 71(MAY):98
Niu TX, Wang XN, Wu HY, Bi JR, Zhang GL (2020) Transcriptomic analysis, motility and biofilm formation characteristics of salmonella typhimurium exposed to benzyl isothiocyanate treatment. Int J Mol Sci 21(3):1025
Asperilla MO, Smego RJ, Scott LK (1990) Quinolone antibiotics in the treatment of Salmonella infections. Rev Infect Dis 12(5):873–889. https://doi.org/10.1093/clinids/12.5.873
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N et al (2013) Antibiotic resistance-the need for global solutions. Lancet Infect Dis 13(12):1057–1098. https://doi.org/10.1016/S1473-3099(13)70318-9
Schoevers EJ, van Leengoed LA, Verheijden JH, Niewold TA (1999) Effects of enrofloxacin on porcine phagocytic function. Antimicrob Agents Chemother 43(9):2138–2143. https://doi.org/10.1128/AAC.43.9.2138
Xie S, Yang F, Tao Y, Chen D, Qu W, Huang L et al (2017) Enhanced intracellular delivery and antibacterial efficacy of enrofloxacin-loaded docosanoic acid solid lipid nanoparticles against intracellular Salmonella. Sci Rep 7:41104. https://doi.org/10.1038/srep41104
Ghosh C, Sarkar P, Issa R, Haldar J (2019) Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol 27(4):323–338. https://doi.org/10.1016/j.tim.2018.12.010
AlKalbani NS, Turner MS, Ayyash MM (2019) Isolation, identification, and potential probiotic characterization of isolated lactic acid bacteria and in vitro investigation of the cytotoxicity, antioxidant, and antidiabetic activities in fermented sausage. Microb Cell Fact 18(1):188. https://doi.org/10.1186/s12934-019-1239-1
Gao J, Li X, Zhang G, Sadiq FA, Simal-Gandara J, Xiao J et al (2021) Probiotics in the dairy industry-advances and opportunities. Compr Rev Food Sci Food Saf 20(4):3937–3982. https://doi.org/10.1111/1541-4337.12755
Haghshenas B, Nami Y, Almasi A, Abdullah N, Radiah D, Rosli R et al (2017) Isolation and characterization of probiotics from dairies. Iran J Microbiol 9(4):234–243
Taye Y, Degu T, Fesseha H, Mathewos M (2021) Isolation and identification of lactic acid bacteria from cow milk and milk products. ScientificWorldJournal 2021:4697445. https://doi.org/10.1155/2021/4697445
O'Toole PW, Cooney JC (2008) Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip Perspect Infect Dis 2008: 175285. https://doi.org/10.1155/2008/175285
Sanders ME (2011) Impact of probiotics on colonizing microbiota of the gut. J Clin Gastroenterol 45(Suppl):S115–S119. https://doi.org/10.1097/MCG.0b013e318227414a
Ganji-Arjenaki M, Rafieian-Kopaei M (2018) Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol 233(3):2091–2103. https://doi.org/10.1002/jcp.25911
Di Marzio L, Russo FP, D’Alo S, Biordi L, Ulisse S, Amicosante G et al (2001) Apoptotic effects of selected strains of lactic acid bacteria on a human T leukemia cell line are associated with bacterial arginine deiminase and/or sphingomyelinase activities. Nutr Cancer 40(2):185–196. https://doi.org/10.1207/S15327914NC402_16
Ma D, Forsythe P, Bienenstock J (2004) Live Lactobacillus rhamnosus [corrected] is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect Immun 72(9):5308–5314. https://doi.org/10.1128/IAI.72.9.5308-5314.2004
Maassen CB, van Holten-Neelen C, Balk F, den Bak-Glashouwer MJ, Leer RJ, Laman JD et al (2000) Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 18(23):2613–2623. https://doi.org/10.1016/s0264-410x(99)00378-3
Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C et al (2004) Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 127(5):1474–1487. https://doi.org/10.1053/j.gastro.2004.09.001
West CE, Jenmalm MC, Prescott SL (2015) The gut microbiota and its role in the development of allergic disease: a wider perspective. Clin Exp Allergy 45(1):43–53. https://doi.org/10.1111/cea.12332
Wang Y, Lv X, Li X, Zhao J, Zhang K, Hao X et al (2021) Protective effect of Lactobacillus plantarum P8 on growth performance, intestinal health, and microbiota in Eimeria-infected broilers. Front Microbiol 12: 705758. https://doi.org/10.3389/fmicb.2021.705758
Wang B, Zhou Y, Mao Y, Gong L, Li X, Xu S et al (2021) Dietary supplementation with Lactobacillus plantarum ameliorates compromise of growth performance by modulating short-chain fatty acids and intestinal dysbiosis in broilers under clostridium perfringens challenge. Front Nutr 8:706148. https://doi.org/10.3389/fnut.2021.706148
Peng Q, Zeng XF, Zhu JL, Wang S, Liu XT, Hou CL et al (2016) Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult Sci 95(4):893–900. https://doi.org/10.3382/ps/pev435
Mazkour S, Shekarforoush SS, Basiri S (2019) The effects of supplementation of Bacillus subtilis and Bacillus coagulans spores on the intestinal microflora and growth performance in rat. Iran J Microbiol 11(3):260–266
Jang YJ, Kim WK, Han DH, Lee K, Ko G (2019) Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 10(6):696–711. https://doi.org/10.1080/19490976.2019.1589281
Wang M, Wu H, Lu L, Jiang L, Yu Q (2020) Lactobacillus reuteri promotes intestinal development and regulates mucosal immune function in newborn piglets. Front Vet Sci 7:42. https://doi.org/10.3389/fvets.2020.00042
Da SL, Pereira OG, Roseira J, Agarussi M, Da SV, Da ST et al (2020) Effect of wild Lactobacillus buchneri strains on the fermentation profile and microbial populations of sugarcane silage. Recent Pat Food Nutr Agric 11(1):63–68. https://doi.org/10.2174/2212798410666190128101343
Heinl S, Wibberg D, Eikmeyer F, Szczepanowski R, Blom J, Linke B et al (2012) Insights into the completely annotated genome of Lactobacillus buchneri CD034, a strain isolated from stable grass silage. J Biotechnol 161(2):153–166. https://doi.org/10.1016/j.jbiotec.2012.03.007
Heinl S, Grabherr R (2017) Systems biology of robustness and flexibility: Lactobacillus buchneri-a show case. J Biotechnol 257:61–69. https://doi.org/10.1016/j.jbiotec.2017.01.007
Oude ES, Krooneman J, Gottschal JC, Spoelstra SF, Faber F, Driehuis F (2001) Anaerobic conversion of lactic acid to acetic acid and 1, 2-propanediol by Lactobacillus buchneri. Appl Environ Microbiol 67(1):125–132. https://doi.org/10.1128/AEM.67.1.125-132.2001
Rabelo CHS, Basso FC, Lara EC, Jorge LGO, Härter CJ, Mari LJ et al (2017) Effects of Lactobacillus buchneri as a silage inoculant or probiotic on in vitro organic matter digestibility, gas production and volatile fatty acids of low dry-matter whole-crop maize silage. Grass Forage Sci 72(3): 534–544. https://doi.org/10.1111/gfs.12273
Schmidt P, Nussio L, Queiroz O, Santos M, Zopollatto M, Filho S et al (2014) Effects of Lactobacillus buchneri on the nutritive value of sugarcane silage for finishing beef bulls. Revista Brasileira de Zootecnia 43(1):8–13. https://doi.org/10.1590/S1516-35982014000100002
Muck RE, Nadeau E, McAllister TA, Contreras-Govea FE, Santos MC, Kung LJ (2018) Silage review: recent advances and future uses of silage additives. J Dairy Sci 101(5):3980–4000. https://doi.org/10.3168/jds.2017-13839
Zielinska K, Fabiszewska A, Stefańska I (2015) Different aspects of Lactobacillus inoculants on the improvement of quality and safety of alfalfa silage. Chilean journal of agricultural research 75(3):298–306. https://doi.org/10.4067/S0718-58392015000400005
Vazquez-Torres A, Jones-Carson J, Baumler AJ, Falkow S, Valdivia R, Brown W et al (1999) Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401(6755):804–808. https://doi.org/10.1038/44593
Organisation WG (2017) World gastroenterology Organisation global guidelines: probiotics and prebiotics.
Binda S, Hill C, Johansen E, Obis D, Pot B, Sanders ME et al (2020) Criteria to qualify microorganisms as “probiotic” in foods and dietary supplements. Front Microbiol 11:1662. https://doi.org/10.3389/fmicb.2020.01662
Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L et al (2006) Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 101(7):1581–1590. https://doi.org/10.1111/j.1572-0241.2006.00734.x
Acurcio LB, Wuyts S, de Cicco SS, Sant’Anna FM, Pedroso S, Bastos RW et al (2020) Milk Fermented by Lactobacillus paracasei NCC 2461 (ST11) Modulates the immune response and microbiota to exert its protective effects against Salmonella typhimurium infection in mice. Probiotics Antimicrob Proteins 12(4):1398–1408. https://doi.org/10.1007/s12602-020-09634-x
Kim WK, Jang YJ, Han DH, Jeon K, Lee C, Han HS et al (2020) Lactobacillus paracasei KBL382 administration attenuates atopic dermatitis by modulating immune response and gut microbiota. Gut Microbes 12(1):1–14. https://doi.org/10.1080/19490976.2020.1819156
Chao A, Bunge J (2002) Estimating the number of species in a stochastic abundance model. Blackwell Publishing Ltd 58(3):531–539
Hill T, Walsh KA, Harris JA, Ett BM (2002) Using ecological diversity measures with bacterial communities. FEMS Microbiol Ecol 43(1):1–11
Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA et al (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31(9):814–821. https://doi.org/10.1038/nbt.2676
Bron PA, Kleerebezem M, Brummer RJ, Cani PD, Mercenier A, MacDonald TT et al (2017) Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 117(1):93–107. https://doi.org/10.1017/S0007114516004037
Bron PA, van Baarlen P, Kleerebezem M (2011) Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 10(1):66–78. https://doi.org/10.1038/nrmicro2690
Wan LY, Chen ZJ, Shah NP, El-Nezami H (2016) Modulation of intestinal epithelial defense responses by probiotic bacteria. Crit Rev Food Sci Nutr 56(16):2628–2641. https://doi.org/10.1080/10408398.2014.905450
Nemcova R, Bomba A, Gancarcikova S, Reiffova K, Guba P, Koscova J et al (2007) Effects of the administration of lactobacilli, maltodextrins and fructooligosaccharides upon the adhesion of E. coli O8:K88 to the intestinal mucosa and organic acid levels in the gut contents of piglets. Vet Res Commun 31(7): 791–800. https://doi.org/10.1007/s11259-007-0048-x
El-Shall NA, Awad AM, El-Hack M, Naiel M, Othman SI, Allam AA et al (2019) The simultaneous administration of a probiotic or prebiotic with live Salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in Salmonella-challenged broilers. Animals (Basel) 10(1):70. https://doi.org/10.3390/ani10010070
Zhang D, Li R, Li J (2012) Lactobacillus reuteri ATCC 55730 and L22 display probiotic potential in vitro and protect against Salmonella-induced pullorum disease in a chick model of infection. Res Vet Sci 93(1):366–373. https://doi.org/10.1016/j.rvsc.2011.06.020
Santos TT, Ornellas R, Acurcio LB, Sandes S, Da CA, Uetanabaro A et al (2021) Differential immune response of Lactobacillus plantarum 286 against salmonella typhimurium infection in conventional and germ-free mice. Adv Exp Med Biol 1323:1–17. https://doi.org/10.1007/5584_2020_544
Saif YM, Barnes HJ, Glasson RJ, Fadly MA, McDougald RL, And Swayne ED (2003) Diseases of poultry, 11th edn. Blackwell Publishing Professional Iowa State Press, USA
Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT et al (2010) Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A 107(7):3070–3075. https://doi.org/10.1073/pnas.0910497107
Restif O, Goh YS, Palayret M, Grant AJ, McKinley TJ, Clark MR et al (2013) Quantification of the effects of antibodies on the extra- and intracellular dynamics of Salmonella enterica. J R Soc Interface 10(79):20120866. https://doi.org/10.1098/rsif.2012.0866
Hassan JO, Barrow PA, Mockett AP, Mcleod S (1990) Antibody response to experimental Salmonella typhimurium infection in chickens measured by ELISA. Vet Rec 126(21):519–522
McClelland M, Sanderson KE, Spieth J, Clifton SW, Latreille P, Courtney L et al (2001) Complete genome sequence of Salmonella enterica serovar typhimurium LT2. Nature 413(6858):852–856. https://doi.org/10.1038/35101614
Ilaria S, Elena Z, Alice, & Bertocchi, et al (2015) A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350(6262):830–834
Tsai CC, Hsih HY, Chiu HH, Lai YY, Liu JH, Yu B et al (2005) Antagonistic activity against Salmonella infection in vitro and in vivo for two Lactobacillus strains from swine and poultry. Int J Food Microbiol 102(2):185–194. https://doi.org/10.1016/j.ijfoodmicro.2004.12.014
Liu Q, Tian H, Kang Y, Tian Y, Li L, Kang X et al (2021) Probiotics alleviate autoimmune hepatitis in mice through modulation of gut microbiota and intestinal permeability. J Nutr Biochem 98:108863. https://doi.org/10.1016/j.jnutbio.2021.108863
Shao Y, Guo Y, Wang Z (2013) beta-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar typhimurium. Poult Sci 92(7):1764–1773. https://doi.org/10.3382/ps.2013-03029
Watson A, Lipina C, McArdle HJ, Taylor PM, Hundal HS (2016) Iron depletion suppresses mTORC1-directed signalling in intestinal Caco-2 cells via induction of REDD1. Cell Signal 28(5):412–424. https://doi.org/10.1016/j.cellsig.2016.01.014
Chichlowski M, Croom WJ, Edens FW, McBride BW, Qiu R, Chiang CC et al (2007) Microarchitecture and spatial relationship between bacteria and ileal, cecal, and colonic epithelium in chicks fed a direct-fed microbial, PrimaLac, and salinomycin. Poult Sci 86(6):1121–1132. https://doi.org/10.1093/ps/86.6.1121
Samanya M, Yamauchi KE (2002) Histological alterations of intestinal villi in chickens fed dried Bacillus subtilis var. natto. Comp Biochem Physiol A Mol Integr Physiol 133(1):95–104. https://doi.org/10.1016/s1095-6433(02)00121-6
Han KJ, Lee JE, Lee NK, Paik HD (2020) Antioxidant and Anti-inflammatory effect of probiotic Lactobacillus plantarum KU15149 derived from Korean homemade diced-radish kimchi. J Microbiol Biotechnol 30(4):591–598. https://doi.org/10.4014/jmb.2002.02052
Bousmaha-Marroki L, Boutillier D, Marroki A, Grangette C (2021) In vitro anti-staphylococcal and anti-inflammatory abilities of Lacticaseibacillus rhamnosus from infant gut microbiota as potential probiotic against infectious women mastitis. Probiotics and Antimicrobial Proteins 13(4):970–981
Jazi V, Mohebodini H, Ashayerizadeh A, Shabani A, Barekatain R (2019) Fermented soybean meal ameliorates Salmonella typhimurium infection in young broiler chickens. Poult Sci 98(11):5648–5660. https://doi.org/10.3382/ps/pez338
Tafazoli F, Magnusson K, Zheng L (2003) Disruption of epithelial barrier integrity by Salmonella enterica serovar typhimurium requires geranylgeranylated proteins. Infect Immun 71(2):872–881. https://doi.org/10.1128/iai.71.2.872-881.2003
赵效南 (2018) 肠炎沙门氏菌致病机制及丁酸梭菌对其拮抗作用的探究., 山东农业大学
夏溪 (2015) 猪抗菌肽PR39抗细菌感染和保护肠道屏障功能的作用及其机制研究., 浙江大学
Lin Z, Zhang YG, Xia Y, Xu X, Jiao X, Sun J (2016) Salmonella enteritidis effector AvrA stabilizes intestinal tight junctions via the JNK pathway. J Biol Chem 291(52):26837–26849. https://doi.org/10.1074/jbc.M116.757393
Bai SP, Huang Y, Luo YH, Wang LL, Ding XM, Wang JP et al (2014) Alteration in lymphocytes responses, cytokine and chemokine profiles in laying hens infected with Salmonella typhimurium. Vet Immunol Immunopathol 160(3–4):235–243. https://doi.org/10.1016/j.vetimm.2014.05.015
Dar MA, Urwat U, Ahmad SM, Ahmad R, Kashoo ZA, Dar TA et al (2019) Gene expression and antibody response in chicken against Salmonella typhimurium challenge. Poult Sci 98(5):2008–2013. https://doi.org/10.3382/ps/pey560
Huang K, Fresno AH, Skov S, Olsen JE (2019) Dynamics and outcome of macrophage interaction between Salmonella gallinarum, Salmonella typhimurium, and Salmonella dublin and macrophages from chicken and cattle. Front Cell Infect Microbiol 9:420. https://doi.org/10.3389/fcimb.2019.00420
Yu J, Zhu YH, Yang GY, Zhang W, Zhou D, Su JH et al (2017) Anti-inflammatory capacity of Lactobacillus rhamnosus GG in monophasic variant Salmonella infected piglets is correlated with impeding NLRP6-mediated host inflammatory responses. Vet Microbiol 210:91–100. https://doi.org/10.1016/j.vetmic.2017.08.008
Beibei L, Xiaoqian H, Ziye Z, Wei C, Die W, Dao X (2022) The role of intestinal epithelial cell HIF-1 α in regulatiing intestinal mucosal barrier function in mice fed with high-fat diet, The 12th National Sports Science Conference (pp. 2). Rizhao, Shandong, China
Zhang F, Li Y, Wang X, Wang S, Bi D (2019) The impact of Lactobacillus plantarum on the gut microbiota of mice with DSS-induced colitis. Biomed Res Int 2019:3921315. https://doi.org/10.1155/2019/3921315
Wang WC, Yan FF, Hu JY, Amen OA, Cheng HW (2018) Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J Anim Sci 96(5):1654–1666. https://doi.org/10.1093/jas/sky092
Kong W, Huang C, Tang Y, Zhang D, Wu Z, Chen X (2017) Effect of Bacillus subtilis on Aeromonas hydrophila-induced intestinal mucosal barrier function damage and inflammation in grass carp (Ctenopharyngodon idella). Sci Rep 7(1):1588
Hirano T, Taga T, Yasukawa K, Nakajima K, Nakano N, Takatsuki F et al (1987) Human B-cell differentiation factor defined by an anti-peptide antibody and its possible role in autoantibody production. Proc Natl Acad Sci U S A 84(1):228–231. https://doi.org/10.1073/pnas.84.1.228
Xu Y, Yu Y, Shen Y, Li Q, Lan J, Wu Y et al (2021) Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult Sci 100(9):101358. https://doi.org/10.1016/j.psj.2021.101358
Wei HX, Wang B, Li B (2020) IL-10 and IL-22 in mucosal immunity: driving protection and pathology. Front Immunol 11:1315. https://doi.org/10.3389/fimmu.2020.01315
Eri RD, Adams RJ, Tran TV, Tong H, Das I, Roche D et al (2011) An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol 4:354–364
Gallo RL, Hooper LV (2012). Epithelial antimicrobial defence of the skin and intestine, Nature reviews. Immunology 12:503–516
Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG (2007) MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med 204(8):1891–1900. https://doi.org/10.1084/jem.20070563
Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O et al (2011) The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334(6053):255–258. https://doi.org/10.1126/science.1209791
Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q et al (2008) Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14(3):282–289. https://doi.org/10.1038/nm1720
Natividad JM, Hayes CL, Motta JP, Jury J, Galipeau HJ, Philip V et al (2013) Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. Appl Environ Microbiol 79(24):7745–7754. https://doi.org/10.1128/AEM.02470-13
Zenilman ME, Magnuson TH, Swinson KL, Egan JM, Perfetti R, Shuldiner AR (1996) Pancreatic thread protein is mitogenic to pancreatic-derived cells in culture. Gastroenterology 110(4):1208–1214
Jha R, Fouhse JM, Tiwari UP, Li L, Willing BP (2019) Dietary fiber and intestinal health of monogastric animals. Frontiers in Veterinary Science 6:48
Preidis GA, Ajami NJ, Wong MC, Bessard BC, Conner ME, Petrosino JF (2015) Composition and function of the undernourished neonatal mouse intestinal microbiome. J Nutr Biochem 26(10):1050–1057. https://doi.org/10.1016/j.jnutbio.2015.04.010
Lin P, Ding B, Feng C, Yin S, Zhang T, Qi X et al (2017) Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J Affect Disord 207:300–304. https://doi.org/10.1016/j.jad.2016.09.051
Mima K, Ogino S, Nakagawa S, Sawayama H, Kinoshita K, Krashima R et al (2017) The role of intestinal bacteria in the development and progression of gastrointestinal tract neoplasms. Surg Oncol 26(4):368–376. https://doi.org/10.1016/j.suronc.2017.07.011
Robinson K, Xiao Y, Johnson TJ, Chen B, Yang Q, Lyu W et al (2020) Chicken intestinal mycobiome: initial characterization and its response to bacitracin methylene disalicylate. Appl Environ Microbiol 86(13):e304–e320. https://doi.org/10.1128/AEM.00304-20
Sun P, Zhang W, Miao Y, Chen Z (2019) Letter: meta-analysis of prebiotics, probiotics, synbiotics and antibiotics in IBS. Aliment Pharmacol Ther 49(9):1253–1254. https://doi.org/10.1111/apt.15230
Wang W, Cao J, Li JR, Yang F, Li Z, Li LX (2016) Comparative analysis of the gastrointestinal microbial communities of bar-headed goose (Anser indicus) in different breeding patterns by high-throughput sequencing. Microbiol Res 182:59–67. https://doi.org/10.1016/j.micres.2015.10.003
Hu JL, Nie SP, Wu QM, Li C, Fu ZH, Gong J et al (2014) Polysaccharide from seeds of Plantago asiatica L. affects lipid metabolism and colon microbiota of mouse. J Agric Food Chem 62(1):229–234. https://doi.org/10.1021/jf4040942
Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444(7122):1022–1023. https://doi.org/10.1038/4441022a
Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F et al (2016) Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail 4(3):220–227. https://doi.org/10.1016/j.jchf.2015.10.009
Khalili H, Chan S, Lochhead P, Ananthakrishnan AN, Hart AR, Chan AT (2018) The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 15(9):525–535. https://doi.org/10.1038/s41575-018-0022-9
Pan S, Zhang K, Ding X, Wang J, Peng H, Zeng Q et al (2018) Effect of high dietary manganese on the immune responses of broilers following oral Salmonella typhimurium inoculation. Biol Trace Elem Res 181(2):347–360. https://doi.org/10.1007/s12011-017-1060-9
Dandachi I, Anani H, Hadjadj L, Brahimi S, Lagier J, Daoud Z et al (2021) Genome analysis of Lachnoclostridium phocaeense isolated from a patient after kidney transplantation in Marseille 41:100863
Ibrahim A, Hugerth LW, Hases L, Saxena A, Seifert M, Thomas Q et al (2019) Colitis-induced colorectal cancer and intestinal epithelial estrogen receptor beta impact gut microbiota diversity. Int J Cancer 144(12):3086–3098. https://doi.org/10.1002/ijc.32037
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y, Keilbaugh SA et al (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108
Lagkouvardos I, Lesker TR, Hitch T, Galvez E, Smit N, Neuhaus K et al (2019) Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 7(1):28. https://doi.org/10.1186/s40168-019-0637-2
Seedorf H, Griffin NW, Ridaura VK, Reyes A, Cheng J, Rey FE et al (2014) Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 159(2):253–266. https://doi.org/10.1016/j.cell.2014.09.008
Yan S, Yang B, Zhao J, Zhao J, Stanton C, Ross RP et al (2019) A ropy exopolysaccharide producing strain Bifidobacterium longum subsp. longum YS108R alleviates DSS-induced colitis by maintenance of the mucosal barrier and gut microbiota modulation. Food Funct 10(3):1595–1608. https://doi.org/10.1039/c9fo00014c
Ma L, Ni Y, Wang Z, Tu W, Ni L, Zhuge F et al (2020) Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 12(1):1–19. https://doi.org/10.1080/19490976.2020.1832857
Hu S, Wang J, Xu Y, Yang H, Wang J, Xue C et al (2019) Anti-inflammation effects of fucosylated chondroitin sulphate from Acaudina molpadioides by altering gut microbiota in obese mice. Food Funct 10(3):1736–1746
Funding
This research was funded by the Natural Science Foundation of Guangxi Province: 2021JJA130237, the Guangxi Key R&D Program: AB21238003, AB21220005-4, the Natural Science Foundation of Guangxi Province: 2021GXNSFAA196058, the National Technical System Construction Project for Waterfowl Industry: CARS-42–55, the Guangxi Broiler Industry Innovation Team Construction Project: nycytxgxcxtd, the Major Science and Technology Project of Liangqing District: 202118, the Key R&D Program of Nanning City: 20212023, the Key R&D Program of Fangchenggang City: AB21014016, the Key R&D Program of Wuming District: 20210102, and the Key R&D Program of Jiangnan District: 20220620–2.
Author information
Authors and Affiliations
Contributions
Conceptualization, Yan Shi, Hao Peng, and Xun Li; formal analysis, Yan Shi, Hao Peng, and Xun Li; investigation, Yan Shi, Hao Peng, Xun Li, Yuying Liao, Jun Li, Yangyan Ying, Hongyan Peng, Leping Wang, Yizhou Tan, Changting Li, Huili Bai, and Chunxia Ma, Wenbao Tan; writing—original draft, Yan Shi; writing—review and editing, Hao Peng and Xun Li. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
All of the experiments were performed in accordance with the guidelines of the regional Animal Ethics Committee and were approved by the Guangxi University Ethical Committee (Project ID GXU2020-069), which are in accordance with the guidelines for the care and use of laboratory animals described by the US National Institutes of Health.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12602_2023_10145_MOESM1_ESM.zip
Supplementary file1 Appendix 1 The pathological changes of liver(a) and spleen(b) and the clinical symptoms of mice. (ZIP 55699 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, Y., Peng, H., Liao, Y. et al. The Prophylactic Protection of Salmonella Typhimurium Infection by Lentilactobacillus buchneri GX0328-6 in Mice. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10145-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10145-8