Abstract
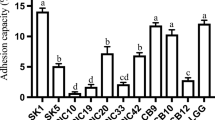
Probiotic microorganisms are incorporated in foods due to their numerous health benefits. We investigated lactic acid bacteria (LAB) and yeasts isolated from goat milk in Nigeria for novel probiotic strains. In this study, a total of 27 LAB and 23 yeast strains were assessed for their probiotic potentials. Only six LAB strains (Weissella cibaria GM 93m3, Weissella confusa GM 92m1, Pediococcus acidilactici GM 18a, Pediococcus pentosaceus GM 23d, Lactiplantibacillus pentosus GM 102s4, Limosilactobacillus fermentum GM 30m1) and four yeast strains (Candida tropicalis 12a, C. tropicalis 33d, Diutina rugosa 53b, and D. rugosa 77a) identified using partial 16S and 26S rDNA sequencing, respectively, showed survival at pH 2.5, 0.3% bile salt, and simulated gastrointestinal conditions and possessed auto-aggregative and hydrophobic properties, thus satisfying key in vitro criteria as probiotics. All LAB strains showed coaggregation properties and antimicrobial activities against pathogens. Pediococcus pentosaceus GM 23d recorded the strongest coaggregation percentage (34–94%) against 14 pathogens, while W. cibaria GM 93m3 showed the least (6–57%) against eight of the 14 pathogens. The whole cell and extracellular extracts of LAB and yeast strains, with the exception of D. rugosa 77a, had either 2,2-diphenyl-1-picryl-hydrazyl and/or hydroxyl radical scavenging activity. In conclusion, all six LAB and four yeast strains are important probiotic candidates that can be further investigated for use as functional starter cultures.











Similar content being viewed by others
Availability of Data and Materials
The data that support the findings of this study are available on request from M.O.A.
References
Kanmani P, Satish Kumar R, Yuvaraj N et al (2013) Probiotics and its functionally valuable products—a review. Crit Rev Food Sci Nutr 53:641–658. https://doi.org/10.1080/10408398.2011.553752
FAO/WHO (2006) Probiotics in food health and nutritional properties and guidelines for evaluation FAO FOOD AND NUTRITION PAPER
Guarner F, Khan AG, Garisch J, Eliakim R, Gangl A, Thomson A, ... Kim N (2012) World gastroenterology organisation global guidelines: probiotics and prebiotics. J Clin Gastro 46(6):468–481. https://doi.org/10.1097/MCG.0b013e3182549092
Soccol CR, Porto L, Vandenberghe DS et al (2013) The potential of probiotics : a review. 48:413–434
Al-Saeed G (2017) Facts about probiotics. J Pediatr Neonatal Care 6:5–7. https://doi.org/10.15406/jpnc.2017.06.00236
Oikonomou G, Addis MF, Chassard C et al (2020) Milk microbiota: what are we exactly talking about? Front Microbiol 11:1–15. https://doi.org/10.3389/fmicb.2020.00060
Akinyemi MO, Ayeni KI, Ogunremi OR et al (2021) A review of microbes and chemical contaminants in dairy products in sub-Saharan Africa. Compr Rev Food Sci Food Saf. https://doi.org/10.1111/1541-4337.12712
Wochner KF, Becker-Algeri TA, Colla E et al (2018) The action of probiotic microorganisms on chemical contaminants in milk. Crit Rev Microbiol 44:112–123. https://doi.org/10.1080/1040841X.2017.1329275
Sakandar HA, Zhang H (2021) Trends in probiotic(s)-fermented milks and their in vivo functionality: a review. Trends Food Sci Technol 110:55–65. https://doi.org/10.1016/j.tifs.2021.01.054
Banwo K, Sanni A, Tan H (2013) Functional properties of Pediococcus species isolated from traditional fermented cereal gruel and milk in Nigeria. Food Biotechnol 27:14–38. https://doi.org/10.1080/08905436.2012.755626
Mbuk EU, Kwaga JKP, Bale JOO, Umoh JU (2016) Molecular identification of yeasts associated with raw cow milk from peri-urban farms in Kaduna State, Nigeria. J Yeast Fungal Res 7:39–46. https://doi.org/10.5897/JYFR2016.0172
Vinderola CG, Reinheimer JA (2003) Lactic acid starter and probiotic bacteria: a comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res Int 36:895–904. https://doi.org/10.1016/S0963-9969(03)00098-X
Ajao O, Banwo K, Ogunremi O, Sanni A (2018) Antimicrobial properties and probiotic potentials of lactic acid bacteria isolated from raw beef in Ibadan, Nigeria. J Microbiol Biotechnol Food Sci 8:770–773. https://doi.org/10.15414/jmbfs.2018.8.2.770-773
Byakika S, Mukisa IM, Mugabi R, Muyanja C (2019) Antimicrobial activity of lactic acid bacteria starters against acid tolerant, antibiotic resistant, and potentially virulent E. coli isolated from a fermented sorghum-millet beverage. Int J Microbiol 2019:2013539. https://doi.org/10.1155/2019/2013539
Jordaan K, Bezuidenhout CC (2016) Bacterial community composition of an urban river in the North West Province, South Africa, in relation to physico-chemical water quality. Environ Sci Pollut Res 23:5868–5880. https://doi.org/10.1007/s11356-015-5786-7
Bories G, Brantom P, Brufau De Barberà J et al (2008) Update of the criteria used in the assessment of bacterial resistance to antibiotics of human or veterinary importance 2 Prepared by the Panel on Additives and Products or Substances used in Animal Feed. EFSA J 732:1–15. https://doi.org/10.2903/j.efsa.2008.732
Uymaz Tezel B (2019) Preliminary In vitro evaluation of the probiotic potential of the bacteriocinogenic strain Enterococcus lactis PMD74 isolated from ezine cheese. J Food Qual 2019:1–12. https://doi.org/10.1155/2019/4693513
Del Re B, Sgorbati B, Miglioli M, Palenzona D (2000) Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 31:438–442. https://doi.org/10.1046/j.1365-2672.2000.00845.x
Makinde OM, Sulyok M, Adeleke RA, Krska RA, Ezekiel CN (manuscript in preparation) Biotoxins and bacterial assessment of ready-to-eat foods vended in Lagos, Nigeria
Ogunremi OR, Sanni AI, Agrawal R (2015) Probiotic potentials of yeasts isolated from some cereal-based Nigerian traditional fermented food products. J Appl Microbiol 119:797–808. https://doi.org/10.1111/jam.12875
Ismail A, Ktari L, Ahmed M et al (2016) Antimicrobial activities of bacteria associated with the brown alga Padina pavonica. Front Microbiol 7:1072. https://doi.org/10.3389/fmicb.2016.01072
Sharma OP, Bhat TK (2009) DPPH antioxidant assay revisited. Food Chem 113:1202–1205. https://doi.org/10.1016/j.foodchem.2008.08.008
He ZS, Luo H, Cao CH, Cui ZW (2004) Photometric determination of hydroxyl free radical in Fenton system by brilliant green. Am J Chinese Clin Med 6:236–237
Addis MF, Tanca A, Uzzau S et al (2016) The bovine milk microbiota: insights and perspectives from -omics studies. Mol Biosyst 12:2359–2372. https://doi.org/10.1039/C6MB00217J
Xiong L, Ni X, Niu L et al (2019) Isolation and preliminary screening of a Weissella confusa Strain from giant panda (Ailuropoda melanoleuca). Probiotics Antimicrob Proteins 11:535–544. https://doi.org/10.1007/s12602-018-9402-2
Son SH, Jeon HL, Yang SJ et al (2017) In vitro characterization of Lactobacillus brevis KU15006, an isolate from kimchi, reveals anti-adhesion activity against foodborne pathogens and antidiabetic properties. Microb Pathog 112:135–141. https://doi.org/10.1016/j.micpath.2017.09.053
Ladha G, Jeevaratnam K (2018) Probiotic potential of Pediococcus pentosaceus LJR1, a bacteriocinogenic strain isolated from rumen liquor of goat (Capra aegagrus hircus). Food Biotechnol 32:60–77. https://doi.org/10.1080/08905436.2017.1414700
Jafari-Nasab T, Khaleghi M, Farsinejad A, Khorrami S (2021) Probiotic potential and anticancer properties of Pediococcus sp. isolated from traditional dairy products. Biotechnol Rep 29:e00593. https://doi.org/10.1016/j.btre.2021.e00593
Yang SJ, Kim K-T, Kim TY, Paik H-D (2020) Probiotic properties and antioxidant activities of Pediococcus pentosaceus SC28 and Levilactobacillus brevis KU15151 in fermented black gamju. Foods 9:1154. https://doi.org/10.3390/foods9091154
Kumara SS, Bashisht A, Venkateswaran G et al (2019) Characterization of novel Lactobacillus fermentum from curd samples of indigenous cows from Malnad Region, Karnataka, for their Aflatoxin B1 binding and probiotic properties. Probiotics Antimicrob Proteins 11:1100–1109. https://doi.org/10.1007/s12602-018-9479-7
Zommiti M, Bouffartigues E, Maillot O et al (2018) In vitro assessment of the probiotic properties and bacteriocinogenic potential of pediococcus pentosaceus MZF16 isolated from artisanal tunisian meat "dried ossban. Front Microbiol 9:2607. https://doi.org/10.3389/fmicb.2018.02607
Soccol CR, de Souza Vandenberghe LP, Spier MR et al (2010) The potential of probiotics: a review. Food Technol Biotechnol 48:413–434
Raccach M (2014) Pediococcus. In: Encyclopedia of Food Microbiology: Second Edition. Elsevier Inc., pp 1–5
Barbosa J, Borges S, Teixeira P (2015) Pediococcus acidilactici as a potential probiotic to be used in food industry. Int J Food Sci Technol 50:1151–1157. https://doi.org/10.1111/ijfs.12768
Abriouel H, Lerma LL, Casado Muñoz MDC et al (2015) The controversial nature of the Weissella genus: technological and functional aspects versus whole genome analysis-based pathogenic potential for their application in food and health. Front Microbiol 6:1197. https://doi.org/10.3389/fmicb.2015.01197
Quijada NM, De Filippis F, Sanz JJ et al (2018) Different Lactobacillus populations dominate in “Chorizo de León” manufacturing performed in different production plants. Food Microbiol 70:94–102. https://doi.org/10.1016/j.fm.2017.09.009
Billot-Klein D, Gutmann L, Sable S et al (1994) Modification of peptidoglycan precursors is a common feature of the low-level vancomycin-resistant VANB-type Enterococcus D366 and of the naturally glycopeptide-resistant species Lactobacillus casei, Pediococcus pentosaceus, Leuconostoc mesenteroides, and Enterococcus gallinarum. J Bacteriol 176:2398–2405
Ammor MS, Flórez AB, Mayo B (2007) Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol 24:559–570
Danielsen M, Simpson PJ, O’connor EB et al (2007) Susceptibility of Pediococcus spp. to antimicrobial agents. J Appl Microbiol 102:384–389
Franz C, Endo A, Abriouel H et al (2014) The genus Pediococcus. Lact acid Bact Biodivers Taxon 359–376
Sharma S, Agarwal N, Verma P (2012) Probiotics: the emissaries of health from microbial world. J Appl Pharm Sci 2:138–143
Faujdar SS, Mehrishi P, Bishnoi S, Sharma A (2016) Role of probiotics in human health and disease : an update. Int J Curr Microbiol App Sci 5:328–344
Bao Y, Zhang Y, Zhang Y et al (2010) Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 21:695–701. https://doi.org/10.1016/j.foodcont.2009.10.010
Abbasiliasi S, Tan JS, Ibrahim TA et al (2012) Isolation of Pediococcus acidilactici Kp10 with ability to secrete bacteriocin-like inhibitory substance from milk products for applications in food industry. BMC Microbiol 12:260. https://doi.org/10.1186/1471-2180-12-260
Ilavenil S, Park HS, Vijayakumar M et al (2015) Probiotic potential of lactobacillus strains with antifungal activity isolated from animal manure. Sci World J 2015:802570. https://doi.org/10.1155/2015/802570
Lakra AK, Domdi L, Hanjon G et al (2020) Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT 125:109261. https://doi.org/10.1016/j.lwt.2020.109261
Prete R, Long SL, Gallardo AL et al (2020) Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci Rep 10:1–11. https://doi.org/10.1038/s41598-020-58069-5
Yamasaki M, Minesaki M, Iwakiri A et al (2020) Lactobacillus plantarum 06CC2 reduces hepatic cholesterol levels and modulates bile acid deconjugation in Balb/c mice fed a high-cholesterol diet. Food Sci Nutr 8:6164–6173. https://doi.org/10.1002/fsn3.1909
Ali SA, Singh P, Tomar SK et al (2020) Proteomics fingerprints of systemic mechanisms of adaptation to bile in Lactobacillus fermentum. J Proteomics 213:103600. https://doi.org/10.1016/j.jprot.2019.103600
Vasiee A, Falah F, Behbahani BA, Tabatabaee-yazdi F (2020) Probiotic characterization of Pediococcus strains isolated from Iranian cereal-dairy fermented product: Interaction with pathogenic bacteria and the enteric cell line Caco-2. J Biosci Bioeng 130:471–479. https://doi.org/10.1016/j.jbiosc.2020.07.002
Lal Sarkar S, Akter Monika S, Kumar Sanyal S, Chandra Roy P (2020) Probiotic potential of Pediococcus acidilactici and Enterococcus faecium isolated from indigenous yogurt and raw goat milk antimicrobial effect of different spices against human oral pathogens. View project Bacteria from gold grains-diversity, functions and applications. View project. Artic Korean J Microbiol Biotechnol. https://doi.org/10.4014/mbl.1912.12009
Barigela A, Bhukya B (2021) Probiotic Pediococcus acidilactici strain from tomato pickle displays anti-cancer activity and alleviates gut inflammation in-vitro. 3 Biotech 11:23. https://doi.org/10.1007/s13205-020-02570-1
Laurencio-Silva M, Arteaga F, Rondón-Castillo AJ et al (2017) In vitro probiotic potential of Lactobacillus spp. strains from the vagina of dairy cows. Pastos Forrajes 40:206–215
Puniya M, Ravinder Kumar M, Panwar H et al (2016) Screening of lactic acid bacteria of different origin for their probiotic potential. J Food Process Technol 7
Lashani E, Davoodabadi A, Dallal MMS (2020) Some probiotic properties of lactobacillus species isolated from honey and their antimicrobial activity against foodborne pathogens. Vet Res Forum 11:121–126. https://doi.org/10.30466/vrf.2018.90418.2188
Fernández-Pacheco P, García-Béjar B, Jiménez-del Castillo M et al (2020) Potential probiotic and food protection role of wild yeasts isolated from pistachio fruits (Pistacia vera). J Sci Food Agric. https://doi.org/10.1002/jsfa.10839
Wang J, Zhang H, Du H et al (2019) Identification and characterization of Diutina rugosa SD-17 for potential use as a probiotic. LWT 109:283–288. https://doi.org/10.1016/j.lwt.2019.04.042
Wang CY, Lin PR, Ng CC, Shyu YT (2010) Probiotic properties of Lactobacillus strains isolated from the feces of breast-fed infants and Taiwanese pickled cabbage. Anaerobe 16:578–585. https://doi.org/10.1016/j.anaerobe.2010.10.003
Lee N, Kwon KY, Oh SK et al (2014) A multiplex PCR assay for simultaneous detection of escherichia coli O157:H7, Bacillus cereus, vibrio parahaemolyticus, salmonella spp., listeria monocytogenes, and staphylococcus aureus in Korean ready-to-eat food. Foodborne Pathog Dis 11:574–580. https://doi.org/10.1089/fpd.2013.1638
Garcia-Cayuela T, Korany AM, Bustos I et al (2014) Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res Int 57:44–50
Cozzolino A, Vergalito F, Tremonte P et al (2020) Preliminary evaluation of the safety and probiotic potential of Akkermansia muciniphila DSM 22959 in comparison with Lactobacillus rhamnosus GG. Microorganisms 8:189. https://doi.org/10.3390/microorganisms8020189
Cruz-Guerrero A, Hernández-Sánchez H, Rodríguez-Serrano G et al (2014) Commercial probiotic bacteria and prebiotic carbohydrates: a fundamental study on prebiotics uptake, antimicrobials production and inhibition of pathogens. J Sci Food Agric 94:2246–2252
Merino L, Trejo FM, De Antoni G, Golowczyc MA (2019) Lactobacillus strains inhibit biofilm formation of Salmonella sp. isolates from poultry. Food Res Int 123:258–265
Gandomi H, Farhangfar A, Akhondzadeh Basti A et al (2019) Auto and co-aggregation, hydrophobicity and adhesion properties of Lactobacillus plantarum strains isolated from Siahmazgi traditional cheese. Food Health 2:1–5
Chervinets Y, Chervinets V, Shenderov B et al (2018) Adaptation and probiotic potential of lactobacilli, isolated from the oral cavity and intestines of healthy people. Probiotics Antimicrob Proteins 10:22–33
Vidhyasagar V, Jeevaratnam K (2013) Evaluation of Pediococcus pentosaceus strains isolated from Idly batter for probiotic properties in vitro. J Funct Foods 5:235–243. https://doi.org/10.1016/j.jff.2012.10.012
Sabir F, Beyatli Y, Cokmus C, Onal-Darilmaz D (2010) Assessment of potential probiotic properties of Lactobacillus spp., Lactococcus spp., and Pediococcus spp. strains isolated from Kefir. J Food Sci 75:M568–M573. https://doi.org/10.1111/j.1750-3841.2010.01855.x
Ilavenil S, Vijayakumar M, Kim DH et al (2016) Assessment of probiotic, antifungal and cholesterol lowering properties of Pediococcus pentosaceus KCC-23 isolated from Italian ryegrass. J Sci Food Agric 96:593–601. https://doi.org/10.1002/jsfa.7128
Fhoula I, Rehaiem A, Najjari A et al (2018) Functional probiotic assessment and in vivo cholesterol-lowering efficacy of Weissella sp. associated with arid lands living-hosts. Biomed Res Int. https://doi.org/10.1155/2018/1654151
Sharma S, Kandasamy S, Kavitake D, Shetty PH (2018) Probiotic characterization and antioxidant properties of Weissella confusa KR780676, isolated from an Indian fermented food. LWT 97:53–60. https://doi.org/10.1016/j.lwt.2018.06.033
Simões LA, Cristina de Souza A, Ferreira I et al (2021) Probiotic properties of yeasts isolated from Brazilian fermented table olives. J Appl Microbiol 131:1983–1997. https://doi.org/10.1111/JAM.15065
Menezes AGT, Ramos CL, Cenzi G et al (2020) Probiotic potential, antioxidant activity, and phytase production of indigenous yeasts isolated from indigenous fermented foods. Probiotics Antimicrob Proteins 12:280–288. https://doi.org/10.1007/s12602-019-9518-z
Ouwehand AC, Salminen SJ (1998) The health effects of cultured milk products with viable and non-viable bacteria. Int Dairy J 8:749–758
Mokoena MP (2017) Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules 22:1255. https://doi.org/10.3390/molecules22081255
Güllüce M, Karadayı M, Barış Ö (2013) Bacteriocins: promising natural antimicrobials. Local Environ 3:6–10
Soltani S, Hammami R, Cotter PD et al (2021) Bacteriocins as a new generation of antimicrobials: toxicity aspects and regulations. FEMS Microbiol Rev 45:fuaa039
Nascimento BL, Delabeneta MF, Rosseto LRB et al (2020) Yeast mycocins: a great potential for application in health. FEMS Yeast Res 20:16. https://doi.org/10.1093/femsyr/foaa016
Shruthi B, Deepa N, Somashekaraiah R et al (2022) Exploring biotechnological and functional characteristics of probiotic yeasts: a review. Biotechnol Rep 34:e00716. https://doi.org/10.1016/j.btre.2022.e00716
Khay EO, Castro LMP, Bernárdez PF et al (2012) Growth of Enterococcus durans E204 producing bacteriocin-like substance in MRS broth: description of the growth and quantification of the bacteriocin-like substance. African J Biotechnol 11:659–665
Siamansouri M, Mozaffari S, Alikhani F (2013) Bacteriocins and lactic acid bacteria. J Biol 2:227–234
Kiymaci ME, Gumustas M, Altanlar N et al (2018) Determination of probiotic abilities and lactic acid content of Pediococcus acidilactici. Curr Anal Chem 15:511–521. https://doi.org/10.2174/1573411014666180912130839
Djide NJN, Asri RM, Djide N (2020) Sourcing new potential bacteriocin-producing bacteria from dangke, ethnic cheese of Enrekang, Indonesia. In: IOP Conference Series: Earth and Environmental Science. IOP Publishing Ltd, p 012035
Manu P, Agyei M (2017) Bacteriocin activity of lactic acid bacteria isolated from Nunu, a Spontaneously Fermented Milk. 67
Kazi TA, Acharya A, Mukhopadhyay BC et al (2022) Plasmid-based gene expression systems for lactic acid bacteria: a review. Microorganisms 10:1132. https://doi.org/10.3390/MICROORGANISMS10061132
Ridlon JM, Kang DJ, Hylemon PB (2006) Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259
Ridlon JM, Harris SC, Bhowmik S et al (2016) Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 7:22–39
Gadhiya D, Patel AR, Prajapati JB (2015) Current trend and future prospective of functional probiotic milk chocolates and related products - a review. Czech J Food Sci 33:295–301. https://doi.org/10.17221/676/2014-CJFS
Kadaikunnan S, Rejiniemon T, Khaled JM et al (2015) In-vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Ann Clin Microbiol Antimicrob 14:9. https://doi.org/10.1186/s12941-015-0069-1
Tan LT-H, Chan K-G, Khan TM et al (2017) Streptomyces sp. MUM212 as a source of antioxidants with radical scavenging and metal chelating properties. Front Pharmacol 8:276. https://doi.org/10.3389/fphar.2017.00276
Kim H, Kim JS, Kim YG et al (2020) Antioxidant and probiotic properties of lactobacilli and bifidobacteria of human origins. Biotechnol Bioprocess Eng 25:421–430. https://doi.org/10.1007/s12257-020-0147-x
Acknowledgements
The authors appreciate the Society for Applied Microbiology (SfAM) in the UK for partly supporting the study through the 2019 Research Support Grant awarded to MOA under the supervision of CNE.
Funding
This study was partly funded by society for applied microbiology (United Kingdom) through the 2019 Research Support Grant.
Author information
Authors and Affiliations
Contributions
M.O.A: conception of study, design of study, sampling and experimentation, data analysis, manuscript preparation, fine-tuning and revision of manuscript, funding acquisition. O.R.O: design of study, fine-tuning and revision of manuscript. R.A.A: fine-tuning and revision of manuscript. C.N.E: conception of study, design of study, fine-tuning and revision of manuscript, funding acquisition, supervision of study.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akinyemi, M.O., Ogunremi, O.R., Adeleke, R.A. et al. Probiotic Potentials of Lactic Acid Bacteria and Yeasts from Raw Goat Milk in Nigeria. Probiotics & Antimicro. Prot. 16, 163–180 (2024). https://doi.org/10.1007/s12602-022-10022-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-10022-w