Abstract
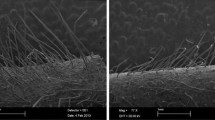
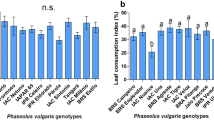
The importance of Spodoptera cosmioides (Lepidoptera: Noctuidae) in soybean crops has been growing, mainly with the cultivation of transgenic Bt plants. The objective of this study was to evaluate antixenosis to S. cosmioides in soybean plants with different leaf colors and trichome densities. Antioxenosis was evaluated by non-preference for feeding and oviposition assays. The study was performed in no-choice and free-choice tests using the genotypes Anta 82, BMX Desafio, BMX Potência, BRS 397, BRS GO 6970 IPRO, BRS 7270 IPRO, BRS 7470 IPRO, BRS 8170 IPRO, BRS 8482, BRS GO 7460, BRS GO Jataí, IAC 100, M 7739 IPRO, NA 5909, NS 7338 IPRO, NS 7447 IPRO, PI 227682 and PI 227687. The genotypes IAC 100, PI 227682 and PI 227687 showed antixenosis to S. cosmioides mediated by leaf color and trichome density. These genotypes could be appropriate for breeding programs aiming to incorporate sources of resistance to S. cosmioides. The cultivar M 7739 IPRO can be used by soybean producers as a control strategy for S. cosmioides due to moderate resistance.



Similar content being viewed by others
References
AGROFIT. (2018). Sistemas de agrotóxicos fitossanitários. Disponível em: <http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons>.
Almeida, A. C. S., Silva, F. C., Almeida, J. A. F. D., & Jesus, F. G. (2017a). Attractiveness and non-preference of soybean cultivars to Heliothis virescens (Lepidoptera: Noctuidae) feeding. Australian Journal of Crop Science, 11, 453–458.
Almeida, A. C. S., Silva, C. L. T., Paiva, L. A., Araujo, M. S., & Jesus, F. G. (2017b). Antibiosis in soybean cultivars to Heliothis virescens (Lepidoptera: Noctuidae) feeding. Florida Entomology, 100, 334–338.
Andrea, M. C. S., Romanelli, T. L., & Molin, J. P. (2016). Energy flows in lowland soybean production system in Brasil. Ciência Rural, 46, 1395–1400.
Ávila, C. J., Vivian, L. M., & Tomquelski, G. V. (2013). Ocorrência, aspectos biológicos, danos e estratégias de manejo de Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) nos sistemas de produção agrícolas. Dourados. Embrapa Agropecuária Oeste, circular técnica, 23, 12 p.
Bernardi, O., Dourado, P. M., Carvalho, R. A., Martinelli, S., Berger, G. U., Head, G. P., & Omoto, C. (2014). High levels of biological activity of Cry1Ac protein expressed on MON 87701 x MON 89788 soybean against Heliothis virescens (Lepidoptera: Noctuidae). Pest Management Scince, 70, 588–594.
Boiça Júnior, A. L., Bottega, D. B., Souza, B. H. S., Rodrigues, N. E. L., & Michelin, V. (2015a). Determinaçion of the resistence tipes to Spodoptera cosmioides (Walker) (Lepidoptera: Nactuidae) in soybean genotupes. Ciencia Agrarias, 36, 607–618.
Boiça Júnior, A. L., Costa, E. N., Souza, B. H. S., & Ribeiro, Z. A. (2015b). Preferência alimentar de adultos de Cerotoma arcuata (Coleoptera: Chrysomelidae) por folhas e vagens de cultivares de soja. Revista da Faculdade de Agronomia, 114, 8–14.
Bortolotto, O. C., Bueno, A. F., Braga, K., Barbosa, G. C., & Sanzovo, A. (2014). Biological characteristics of Heliothis virescens fed with Bt-soybean MON 87701 x MON 89788 and its conventional isoline. Anais da Academia Brasileira Ciência, 86, 973–980.
Bueno, R. C. O. F., Bueno, A. F., Moscardi, F., Parra, J. R. P., & Hoffmann-Campo, C. L. (2011). Lepidopteran larva consumption of soybean foliage: Basis for developing multiple-species economic thresholds for pest management decisions. Pest Management Scince, 67, 170–174.
Bueno, R. C. O. F., Parra, J. R. P., & Bueno, A. F. (2012). Trichogramma pretiosum parasitism of Pseudoplusia includens and Anticarsia gemmatalis eggs at different temperatures. Biological Control, 60, 154–162.
Carvalho, A. L., Antunes, C. H., Freire, F., & Henriques, C. O. (2015). A hybrid input-output multi-objective model to assess economic-energy-environment trade-offs in Brazil. Energy, 182, 769–785.
Coelho, S. A. M. P., Lourenção, A. L., Melo, A. M. T., & Schammass, E. A. (2009). Resistência de genótipos de meloeiro a Bemisia tabaci biótipo B. Bragantia, 68, 1025–1035.
Connor, E. F. (2006). Effects of the light environment on oviposition preference and survival of a leaf-mining moth, Cameraria hamadryadella (Lepidoptera: Gracillariidae), on Quercus alba L. Ecological Entomology, 31, 179–184.
Costa, E. N., Ribeiro, Z. A., Souza, B. H. S., & Boiça Júnior, A. L. (2014). Oviposition preference assessment of Diabrotica speciosa (Coleoptera: Chrysomelidae) for different soybean genotypes. International Journal of Pest Management, 60, 52–58.
Cruz, P. L., Baldin, E. L. L., Guimarães, L. R. P., Pannuti, L. E. R., Lima, G. P. P., Heng-Moss, T. M., & HUNT, T. E. (2016). Tolerance of KS-4202 soybean to the atack of Bemisia tabaci B (Hemiptera: Aleyrodidae). Florida Entomologist, 99, 600–607.
Greene, G. L., Leppla, N. C., & Diekerson, W. A. (1976). Velveatben caterpillar rearing produce and artificial medium. Journal of Economic Entomology, 69, 487–488.
Handley, R., Ekbom, B., & Agren, J. (2005). Variation in trichome density and resistance against a specialist insect herbivore in natural populations of Arabidopsis thaliana. Ecological Entomology, 30, 284–292.
Hilker, M., & Meiners, T. (2011). Plants and insect eggs: How do they affect each other? Phytochemistry, 72, 1612–1623.
Hoffmann-Campo, C. B., Mazzarin, R. M., & Lustosa, P. R. (1994). Mecanismos de resistência de genótipos de soja: teste de não preferência para Anticarsia gemmatalis Hübner, 1818 (Lep.: Noctuidae). Pesquisa Agropecuária Brasileira, 29, 513–519.
Hoffmann-Campo, C. B., Harborne, J. B., & Mccaffery, A. R. (2001). Pre-ingestive and posting estive effects of soybean extracts and rutin on Trichoplusia ni growth. Entomologia Experimentalis et Applicata, 98, 181–194.
Hulburt, D. J., Boerma, H. R., & All, J. N. (2004). Effect of pubescence tip on soybean resistance to lepidopteran insects. Journal of Economic Entomology, 97, 621–627.
Inbar, M., & Gerling, D. (2008). Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annual Review of Entomology, 53, 431–448.
Kogan, M., & Goeden, R. D. (1970). The host-plant range of Lema Trilineata daturaphila (Coleoptera: Chrysomelidae). Annals of the Entomological Society of America, 63, 1175–1180.
Lara, F. M. (1991). Princípios de resistência de plantas a insetos (p. 336). São Paulo: Ícone.
Lenteren, J. C. V., & Noldus, P. J. J. (1990). Whitefly-plant relationships: Behavioral and ecological aspects. In D. Gerling (Ed.), Whiteflies: Their bionomics, pest status and management (pp. 47–89). Wimborne: Intercept.
Lima, A. C. S., & Lara, F. M. (2004). Resistência de genótipos de soja à mosca branca Bemisia tabaci (Genn.) biótipo B (Hemiptera: Aleyrodidae). Neotropical Entomology, 33, 71–75.
Minolta, K. (1998). Precise color communication: Color control from perception to instrumentation. Osaka: Konica Minolta Sensing Inc.
Panizzi, A. R., Bueno, A. F., & Silva, F. A. C. (2012). Insetos que atacam vagens e grãos. In C. B. Hoffmann-Campo, B. S. Corrêa-Ferreira, & F. Moscardi (Eds.), Soja: manejo integrado de insetos e outros artrópodes-praga (pp. 335–420). Embrapa: Brasília.
Piubelli, G. C., Campo, C. B. H., Arruda, I. C., Franchini, J. C., & Lara, F. M. (2003). Flavonoid increase in soybean genotypes as response of Nezara viridula injury and its effect on insect- feeding preference. Journal of Chemical Ecology, 29, 1223–1233.
Prado, J. C., Penaflor, M. F. G. V., Cia, E., Vieira, S. S., Silva, K. I., Carlini-Garcia, L. A., & Lourenção, A. L. (2016). Resistance of cotton genotypes with different leaf colour and trichome density to Bemisia tabaci biotype B. Journal of Applied Entomology, 140, 405–413.
R Development Core Team. (2017). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Reynolds, G. W., Smith, C. M., & Kester, K. M. (1984). Reductions in consumption, utilization, and growth rate of soybean looper (Lepidoptera: Noctuidae) larvae fed foliage of soybean genotype PI 227687. Journal of Economic Entomology, 77, 1371–1375.
Santos, W. J. (2007). Manejo das pragas do algodão com destaque para o cerrado brasileiro. In E. C. Freire (Ed.), Algodão no cerrado do Brasil (pp. 403–478). Associação Brasileira dos Produtores de Algodão: Brasília.
Santos, K. B., Neves, P. J., Meneguim, A. M., Santos, R. B., Santos, W. J., Villas Boas, G., Dumas, V., Martins, E., Praça, L. B., Queiroz, P., Berry, C., & Monnerat, R. (2009). Selection and characterization of the Bacillus thuringiensis strains toxic to Spodoptera eridania (Cramer), Spodoptera cosmiodes (Walker) and Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Biological Control, 50, 157–163.
Santos, K. B., Meneguim, A. M., Santos, W. J., Neves, P. M. O. J., & Santos, R. B. (2010). Characterization of the damage of Spodoptera eridania (Cramer) and Spodoptera cosmioides (Walker) (Lepidoptera: Noctuidae) to structures of cotton plants. Neotropical Entomology, 39, 626–631.
Schlink-Souza, E. C., Baldin, E. L. L., Morando, R., & Lourenção, A. L. (2017). Antixenosis to Chrysodeixis includens (Lepidoptera: Noctuidae) among soybean genotypes. Bragantia, 77, 1–10.
Seifi, A., Visser, R. G. F., & Yuling, B. A. (2013). How to effectively deploy plant resistances to pests and pathogens in crop breeding. Euphytica, 190, 321–334.
Silva, D. M., Hoffmann-Campo, C. B., Bueno, A. F., Bueno, R. C. F., Oliveira, M. C., & Moscardi, F. (2012). Biological characteristics of Anticarsia gemmatalis (Lepidoptera: Noctuidae) for three consecutive generations under different temperatures: Understanding the possible impact of global warning on a soybean pest. Bulletin of Entomological Research, 102, 285–292.
Silva, J. P. G. F., Baldin, E. L. L., Canassa, V. F., Souza, E. S., & Lourenção, A. L. (2014). Assessing antixenosis of soybean entries against Piezodorus guildinii (Hemiptera: Pentatomidae). Arthropod-Plant Interactions, 8, 349–359.
Smith, C. M. (2005). Plant resistance to arthropods: Molecular and conventional approaches (p. 423). Berlin: Springer.
Sosa-Gomez, D. R., & Silva, J. J. (2010). Neotropical brown stink bug (Euschistus heros) resistance to methamidophos in Paraná. Pesquisa Agropecuária Brasileira, 45, 767–769.
Sousa, D. M. G., & Lobato, E. (2004). Cerrado: correção do solo e adubação (2.ed ed.p. 416). Brasília: Embrapa Informação Tecnológica.
Souza, B. H. S., Boiça Junior, A. L., Janini, J. C., Silva, A. G., & Rodrigues, N. E. L. (2012). Feeding of Spodoptera eridania (Lepidoptera: Noctuidae) on soybean genotypes. Revista Colombiana de Entomologia, 38, 215–223.
Souza, P. V., Machado, B. R., Silva, D. C., Menezes, I. P. P., Araújo, M. S., & Jesus, F. G. (2014). Effect of resistance and trichome inducers on attraction of Euchistus heros (Hemiptera: Pentatomidae) to soybeans. African Journal of Agriculture Research, 9, 889–894.
Valle, G. E., & Lourenção, A. L. (2002). Resistência de genótipos de soja a Bemisia tabaci biótipo B (Hemiptera: Aleyrodidae). Neotropical Entomology, 31, 285–295.
Valle, G. E., Lourenção, A. L., & Pinheiro, J. B. (2012). Adult attractiveness and oviposition preference of Bemisia tabaci biotype B in soybean genotypes with different trichome density. Journal of Pest Science, 85, 431–442.
Vieira, S. S., Bueno, A. F., Bueno, R. C. O. F., & Hoffman-Campo, C. B. (2011). Resistance of soybean genotypes to Bemisia tabaci (Genn.) biotype B (Hemiptera: Aleyrodidae). Neotropical Entomology, 40, 117–122.
Acknowledgments
This study was partially supported by a National Council of Research and Technology of Brazil (CNPq). We also recognize the Instituto Federal Goiano for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Queiroz, E.B., da Silva, F.C., Junior, C.B. et al. Antixenosis in soybean to Spodoptera cosmioides (Lepidoptera: Noctuidae) mediated by leaf color and trichome density. Phytoparasitica 48, 813–821 (2020). https://doi.org/10.1007/s12600-020-00840-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-020-00840-5