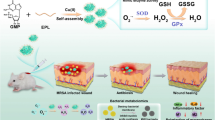
Abstract
Nanoenzyme-mediated antibacterial strategies have been widely exploited to overcome the shortcomings (such as drug resistance and mild-to-severe side effects) of antibiotic therapy. The peroxidase-like activity of nanoenzymes possesses great potential against bacterial infection by the generation of hydroxyl radical (·OH) in the specific microenvironment. However, the lifetime of ·OH is extremely short, and a large amount of the ·OH generated within the infection microenvironment cannot come into contact with bacteria quickly enough, thus resulting in low treatment efficiency. Here, chitosan-oligosaccharide-modified CuS nanoparticles possessing positive charges (PCuS NPs) were prepared using a one-pot method. PCuS NPs exhibited efficient peroxidase-like activity. Importantly, the PCuS NPs can combine with bacteria via electrostatic attraction. The direct contact between the PCuS NPs and bacteria enabled the generation of ·OH in situ on the bacterial surface, ultimately leading to a high antibacterial efficacy at a low concentration in the presence of H2O2. At an effective antibacterial concentration, the PCuS NPs exhibited high cytocompatibility. Furthermore, in vivo results revealed that PCuS NPs not only decreased the size of abscesses but also reduced inflammation and promoted collagen fiber formation. Therefore, PCuS NPs possess great potential against bacterial infection via in situ ·OH generation based on electrostatic attraction.
Graphical abstract

摘要
为了克服抗生素治疗中存在的耐药性和中度至重度副作用等弊端,纳米酶介导的抗菌策略得到广泛的应用。通过 在特定微环境中产生羟基自由基(·OH),具有类过氧化物酶活性的纳米酶在治疗细菌感染中具有巨大的潜力。 然而,·OH 的寿命非常短,导致在微环境中产生的大量·OH 无法足够快速地接触细菌,因此治疗效率偏低。在本 研究中,通过一步法制备了带正电荷的壳寡糖修饰的CuS 纳米颗粒(PCuS NPs)。PCuS NPs 具有高效的类过氧 化物酶活性。重要的是,PCuS NPs 能够通过静电吸引与细菌结合。PCuS NPs 和细菌的直接接触能够使·OH 在细 菌表面原位产生,最终在低浓度H2O2 存在下呈现高的抗菌效率。在有效抗菌浓度下,PCuS NPs 具有良好的细胞 相容性。此外,体内结果表明,PCuS NPs 不仅能减小脓肿的尺寸,而且还能减轻炎症并促进胶原纤维的形成。综 上所述,PCuS NPs 能够通过静电吸引在细菌表面原位生成·OH,具有高的抗细菌感染的应用潜力。






Similar content being viewed by others
References
Petrovic Fabijan A, Lin RC, Ho J, Maddocks S, Ben Zakour NL, Iredell JR. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol. 2020;5(3):465. https://doi.org/10.1038/s41564-019-0634-z.
Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603. https://doi.org/10.1128/CMR.00134-14.
Boldock E, Surewaard BG, Shamarina D, Na M, Fei Y, Ali A, Williams A, Pollitt EJ, Szkuta P, Morris P. Human skin commensals augment Staphylococcus aureus pathogenesis. Nat Microbiol. 2018;3(8):881. https://doi.org/10.1038/s41564-018-0198-3.
Smith PA, Koehler MF, Girgis HS, Yan D, Chen Y, Chen Y, Crawford JJ, Durk MR, Higuchi RI, Kang J. Optimized arylomycins are a new class of Gram-negative antibiotics. Nature. 2018;561(7722):189. https://doi.org/10.1038/s41586-018-0483-6.
Peyrusson F, Varet H, Nguyen TK, Legendre R, Sismeiro O, Coppée JY, Wolz C, Tenson T, Van Bambeke F. Intracellular Staphylococcus aureus persisters upon antibiotic exposure. Nat Commun. 2020;11(1):1. https://doi.org/10.1038/s41467-020-15966-7.
Xie C, Zhang Q, Li Z, Ge S, Ma B. Sustained and microenvironment-accelerated release of minocycline from alginate injectable hydrogel for bacteria-infected wound healing. Polymers. 2022;14(9):1816. https://doi.org/10.3390/polym14091816.
Marchant J. When antibiotics turn toxic. Nature. 2018;555(7697):431. https://doi.org/10.1038/d41586-018-03267-5.
Couce A, Blázquez J. Side effects of antibiotics on genetic variability. FEMS Microbiol Rev. 2009;33(3):531. https://doi.org/10.1111/j.1574-6976.2009.00165.x.
Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet. 2019;393(10167):183. https://doi.org/10.1016/S0140-6736(18)32218-9.
Chin W, Zhong G, Pu Q, Yang C, Lou W, De Sessions PF, Periaswamy B, Lee A, Liang ZC, Ding X. A macromolecular approach to eradicate multidrug resistant bacterial infections while mitigating drug resistance onset. Nat Commun. 2018;9(1):1. https://doi.org/10.1038/s41467-018-03325-6.
Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S, Benito N, Grau S. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev. 2019;32(4):e00031. https://doi.org/10.1128/CMR.00031-19.
Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903. https://doi.org/10.2147/IDR.S234610.
Makabenta JMV, Nabawy A, Li CH, Schmidt-Malan S, Patel R, Rotello VM. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19(1):23. https://doi.org/10.1038/s41579-020-0420-1.
Rubey KM, Brenner JS. Nanomedicine to fight infectious disease. Adv Drug Deliver Rev. 2021;179:113996. https://doi.org/10.1016/j.addr.2021.113996.
Xiu W, Shan J, Yang K, Xiao H, Yuwen L, Wang L. Recent development of nanomedicine for the treatment of bacterial biofilm infections. View. 2021;2(1):20200065. https://doi.org/10.1002/VIW.20200065.
Chai MZ, An MW, Zhang XY, Chu PK. In vitro and in vivo antibacterial activity of graphene oxide-modified porous TiO2 coatings under 808-nm light irradiation. Rare Met. 2022;41(2):540. https://doi.org/10.1007/s12598-021-01754-9.
Ding M, Zhao W, Song LJ, Luan SF. Stimuli-responsive nanocarriers for bacterial biofilm treatment. Rare Met. 2022;41(2):482. https://doi.org/10.1007/s12598-021-01802-4.
Wu M, Zhang Z, Liu Z, Zhang J, Zhang Y, Ding Y, Huang T, Xiang D, Wang Z, Dai Y, Wan X, Wang S, Qian H, Sun Q, Li L. Piezoelectric nanocomposites for sonodynamic bacterial elimination and wound healing. Nano Today. 2021;37:101104. https://doi.org/10.1016/j.nantod.2021.101104.
Liang Y, Liang Y, Zhang H, Guo B. Antibacterial biomaterials for skin wound dressing. Asian J Pharm Sci. 2022;17(3):353. https://doi.org/10.1016/j.ajps.2022.01.001.
Maleki A, He J, Bochani S, Nosrati V, Shahbazi MA, Guo B. Multifunctional photoactive hydrogels for wound healing acceleration. ACS Nano. 2021;15(12):18895. https://doi.org/10.1021/acsnano.1c08334.
Mei L, Zhu S, Liu Y, Yin W, Gu Z, Zhao Y. An overview of the use of nanozymes in antibacterial applications. Chem Eng J. 2021;418:129431. https://doi.org/10.1016/j.cej.2021.129431.
Vallabani NVS, Vinu A, Singh S, Karakoti A. Tuning the ATP-triggered pro-oxidant activity of iron oxide-based nanozyme towards an efficient antibacterial strategy. J Colloid Interf Sci. 2020;567:154. https://doi.org/10.1016/j.jcis.2020.01.099.
Karim MN, Singh M, Weerathunge P, Bian P, Zheng R, Dekiwadia C, Ahmed T, Walia S, Della Gaspera E, Singh S. Visible-light-triggered reactive-oxygen-species-mediated antibacterial activity of peroxidase-mimic CuO nanorods. ACS Appl Nano Mater. 2018;1(4):1694. https://doi.org/10.1021/acsanm.8b00153.
Wang C, Xiao Y, Zhu W, Chu J, Xu J, Zhao H, Shen F, Peng R, Liu Z. Photosensitizer-modified MnO2 nanoparticles to enhance photodynamic treatment of abscesses and boost immune protection for treated mice. Small. 2020;16(28):2000589. https://doi.org/10.1002/smll.202000589.
Yang N, Guo H, Cao C, Wang X, Song X, Wang W, Yang D, Xi L, Mou X, Dong X. Infection microenvironment-activated nanoparticles for NIR-II photoacoustic imaging-guided photothermal/chemodynamic synergistic anti-infective therapy. Biomaterials. 2021;275:120918. https://doi.org/10.1016/j.biomaterials.2021.120918.
Qi Y, Ren S, Ye J, Tian Y, Wang G, Zhang S, Du L, Li Y, Che Y, Ning G. Infection microenvironment-activated core-shell nanoassemblies for photothermal/chemodynamic synergistic wound therapy and multimodal imaging. Acta Biomater. 2022;143:445. https://doi.org/10.1016/j.actbio.2022.02.034.
Wang D, Peng F, Li J, Qiao Y, Li Q, Liu X. Butyrate-inserted Ni–Ti layered double hydroxide film for H2O2-mediated tumor and bacteria killing. Mater Today. 2017;20(5):238. https://doi.org/10.1016/j.mattod.2017.05.001.
Lv R, Liang YQ, Li ZY, Zhu SL, Cui ZD, Wu SL. Flower-like CuS/graphene oxide with photothermal and enhanced photocatalytic effect for rapid bacteria-killing using visible light. Rare Met. 2022;41(2):639. https://doi.org/10.1007/s12598-021-01759-4.
Ding Y, Yuan Z, Hu JW, Xu K, Wang H, Liu P, Cai KY. Surface modification of titanium implants with micro-nano-topography and NIR photothermal property for treating bacterial infection and promoting osseointegration. Rare Met. 2022;41(2):673. https://doi.org/10.1007/s12598-021-01830-0.
Geng H, Pan Y, Zhang R, Gao D, Wang Z, Li B, Li N, Guo D, Xing C. Binding to amyloid-β protein by photothermal blood-brain barrier-penetrating nanoparticles for inhibition and disaggregation of fibrillation. Adv Funct Mater. 2021;31(41):2102953. https://doi.org/10.1002/adfm.202102953.
Jia Q, Song Q, Li P, Huang W. Rejuvenated photodynamic therapy for bacterial infections. Adv Healthc Mater. 2019;8(14):1900608. https://doi.org/10.1002/adhm.201900608.
Hu WC, Younis MR, Zhou Y, Wang C, Xia XH. In situ fabrication of ultrasmall gold nanoparticles/2D MOFs hybrid as nanozyme for antibacterial therapy. Small. 2020;16(23):2000553. https://doi.org/10.1002/smll.202000553.
Ge C, Wu R, Chong Y, Fang G, Jiang X, Pan Y, Chen C, Yin JJ. Synthesis of Pt hollow nanodendrites with enhanced peroxidase-like activity against bacterial infections: implication for wound healing. Adv Healthc Mater. 2018;28(28):1801484. https://doi.org/10.1002/adfm.201801484.
Gligorovski S, Strekowski R, Barbati S, Vione D. Environmental implications of hydroxyl radicals (·OH). Chem Rev. 2015;115(24):13051. https://doi.org/10.1021/cr500310b.
Hou JT, Zhang M, Liu Y, Ma X, Duan R, Cao X, Yuan F, Liao YX, Wang S, Ren WX. Fluorescent detectors for hydroxyl radical and their applications in bioimaging: a review. Coordin Chem Rev. 2020;421:213457. https://doi.org/10.1016/j.ccr.2020.213457.
Berne C, Ducret A, Hardy GG, Brun YV. Adhesins involved in attachment to abiotic surfaces by Gram-negative bacteria. Microbiol Spectr. 2015. https://doi.org/10.1128/microbiolspec.MB-0018-2015.
Simpson BW, Trent MS. Pushing the envelope: LPS modifications and their consequences. Nat Rev Microbiol. 2019;17(7):403. https://doi.org/10.1038/s41579-019-0201-x.
Adekanmbi EO, Giduthuri AT, Waymire S, Srivastava SK. Utilization of dielectrophoresis for the quantification of rare earth elements adsorbed on cupriavidus necator. ACS Sustain Chem Eng. 2020;8(3):1353. https://doi.org/10.1021/acssuschemeng.9b03878.
Zhang C, Fu YY, Zhang X, Yu C, Zhao Y, Sun SK. BSA-directed synthesis of CuS nanoparticles as a biocompatible photothermal agent for tumor ablation in vivo. Dalton T. 2015;44(29):13112. https://doi.org/10.1039/c5dt01467k.
Pan Q, Peng X, Cun JE, Li J, Pu Y, He B. In-situ drug generation and controllable loading: rational design of copper-based nanosystems for chemo-photothermal cancer therapy. Chem Eng J. 2021;409:128222. https://doi.org/10.1016/j.cej.2020.128222.
Yi X, Chen L, Chen J, Maiti D, Chai Z, Liu Z, Yang K. Biomimetic copper sulfide for chemo-radiotherapy: enhanced uptake and reduced efflux of nanoparticles for tumor cells under ionizing radiation. Adv Funct Mater. 2018;28(9):1705161. https://doi.org/10.1002/adfm.201705161.
Xiao Y, Su D, Wang X, Wu S, Zhou L, Shi Y, Fang S, Cheng HM, Li F. CuS microspheres with tunable interlayer space and micropore as a highrate and long-life anode for sodium-ion batteries. Adv Energy Mater. 2018;8(22):1800930. https://doi.org/10.1002/aenm.201800930.
Sarkar AK, Bediako JK, Choi JW, Yun YS. Functionalized magnetic biopolymeric graphene oxide with outstanding performance in water purification. NPG Asia Mater. 2019;11(1):1. https://doi.org/10.1038/s41427-018-0104-8.
Li PC, Liao GM, Kumar SR, Shih CM, Yang CC, Wang DM, Lue SJ. Fabrication and characterization of chitosan nanoparticle-incorporated quaternized poly (vinyl alcohol) composite membranes as solid electrolytes for direct methanol alkaline fuel cells. Electrochim Acta. 2016;187:616. https://doi.org/10.1016/j.electacta.2015.11.117.
Zheng W, Yang Z, Yang J, Qu W, Feng Y, Jiang S, Zhao S, Shih K, Li H. Favorably adjusting the pore characteristics of copper sulfide by template regulation for vapor-phase elemental mercury immobilization. J Mater Chem A. 2022;10(19):10729. https://doi.org/10.1039/D2TA00022A.
Yuan M, Guo X, Pang H. Derivatives (Cu/CuO, Cu/Cu2O, and CuS) of Cu superstructures reduced by biomass reductants. Mater Today Chem. 2021;21:100519. https://doi.org/10.1016/j.mtchem.2021.
Ma B, Wang S, Liu F, Zhang S, Duan J, Li Z, Kong Y, Sang Y, Liu H, Bu W, Li L. Self-assembled copper-amino acid nanoparticles for in situ glutathione “AND” H2O2 sequentially triggered chemodynamic therapy. J Am Chem Soc. 2019;141(2):849. https://doi.org/10.1021/jacs.8b08714.
Dai X, Chen L, Li Z, Li X, Wang J, Hu X, Zhao L, Jia Y, Sun SX, Wu Y, He Y. CuS/KTa0.75Nb0.25O3 nanocomposite utilizing solar and mechanical energy for catalytic N2 fixation. J Colloid Interfaces Sci. 2021;603:220. https://doi.org/10.1016/j.jcis.2021.06.107.
Guo J, Tian H, He J. Integration of CuS nanoparticles and cellulose fibers towards fast, selective and efficient capture and separation of mercury ions. Chem Eng J. 2021;408:127336. https://doi.org/10.1016/j.cej.2020.127336.
Cao J, Sun Q, Shen AG, Fan B, Hu JM. Nano Au@Cu2-xS with near-infrared photothermal and peroxidase catalytic activities redefines efficient antibiofilm-oriented root canal therapy. Chem Eng J. 2021;422:130090. https://doi.org/10.1016/j.cej.2021.130090.
Jana D, Zhao Y. Strategies for enhancing cancer chemodynamic therapy performance. Exploration. 2022;2(2):20210238. https://doi.org/10.1002/exp.20210238.
Wu S, Xu C, Zhu Y, Zheng L, Zhang L, Hu Y, Yu B, Wang Y, Xu FJ. Biofilm-sensitive photodynamic nanoparticles for enhanced penetration and antibacterial efficiency. Adv Funct Mater. 2021;31(33):2103591. https://doi.org/10.1002/adfm.202103591.
Huang Y, Zou L, Wang J, Jin Q, Ji J. Stimuli-responsive nanoplatforms for antibacterial applications. Wires Nanomed Nanobi. 2022;14(3):e1775. https://doi.org/10.1002/wnan.1775.
Li Z, Liu W, Ni P, Zhang C, Wang B, Duan G, Chen C, Jiang Y, Lu Y. Carbon dots confined in N-doped carbon as peroxidase-like nanozyme for detection of gastric cancer relevant D-amino acids. Chem Eng J. 2022;428:131396. https://doi.org/10.1016/j.cej.2021.13.
Jiang B, Duan D, Gao L, Zhou M, Fan K, Tang Y, Xi J, Bi Y, Tong Z, Gao GF. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat Protoc. 2018;13(7):1506. https://doi.org/10.1038/s41596-018-0001-1.
Ji S, Jiang B, Hao H, Chen Y, Dong J, Mao Y, Zhang Z, Gao R, Chen W, Zhang R. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat Catal. 2021;4(5):407. https://doi.org/10.1038/s41929-021-00609-x.
Wei F, Cui X, Wang Z, Dong C, Li J, Han X. Recoverable peroxidase-like Fe3O4@MoS2-Ag nanozyme with enhanced antibacterial ability. Chem Eng J. 2021;408:127240. https://doi.org/10.1016/j.cej.2020.127240.
Gao L, Fan K, Yan X. Iron oxide nanozyme: a multifunctional enzyme mimetic for biomedical applications. Theranostics. 2017;7(13):3207. https://doi.org/10.7150/thno.19738.
Bai Q, Liang M, Wu W, Zhang C, Li X, Liu M, Yang D, Yu WW, Hu Q, Wang L, Du F, Sui N, Zhu Z. Plasmonic nanozyme of graphdiyne nanowalls wrapped hollow copper sulfide nanocubes for rapid bacteria-killing. Adv Funct Mater. 2022;32(20):2112683. https://doi.org/10.1002/adfm.202112683.
Borthakur P, Boruah PK, Das P, Das MR. CuS nanoparticles decorated MoS2 sheets as an efficient nanozyme for selective detection and photocatalytic degradation of hydroquinone in water. New J Chem. 2021;45(19):8714. https://doi.org/10.1039/d1nj00856k.
Borthakur P, Boruah PK, Das MR. Facile synthesis of CuS nanoparticles on two-dimensional nanosheets as efficient artificial nanozyme for detection of ibuprofen in water. J Environ Chem Eng. 2021;9(1):104635. https://doi.org/10.1016/j.jece.2020.104635.
Swaidan A, Borthakur P, Boruah PK, Das MR, Barras A, Hamieh S, Toufaily J, Hamieh T, Szunerits S, Boukherroub R. A facile preparation of CuS-BSA nanocomposite as enzyme mimics: application for selective and sensitive sensing of Cr(VI) ions. Sens Actuators B-Chem. 2019;294:253. https://doi.org/10.1016/j.snb.2019.05.052.
Zhan Y, Zeng Y, Li L, Guo L, Luo F, Qiu B, Huang Y, Lin Z. Cu2+-modified boron nitride nanosheets-supported subnanometer gold nanoparticles: an oxidase-mimicking nanoenzyme with unexpected oxidation properties. Anal Chem. 2020;92(1):1236. https://doi.org/10.1021/acs.analchem.9b04384.
Wang S, Zhao J, Zhang L, Zhang C, Qiu Z, Zhao S, Huang Y, Liang H. A unique multifunctional nanoenzyme tailored for triggering tumor microenvironment activated NIR-II photoacoustic imaging and chemodynamic/photothermal combined therapy. Adv Healthc Mater. 2022;11(3):2102073. https://doi.org/10.1002/adhm.202102073.
Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. 2011;11(8):505. https://doi.org/10.1038/nri3010.
Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O’Connell RM, Iwakura Y, Cheung AL, Cheng G. Inflammasome-mediated production of IL-1β is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179(10):6933. https://doi.org/10.4049/jimmunol.179.10.6933.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 82100974), the Natural Science Foundation of Shandong Province (No. ZR2021QH241), and Qilu Young Scholars Program of Shandong University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Z., Xie, CJ., Ren, XW. et al. CuS nanoenzyme against bacterial infection by in situ hydroxyl radical generation on bacteria surface. Rare Met. 42, 1899–1911 (2023). https://doi.org/10.1007/s12598-022-02223-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02223-7