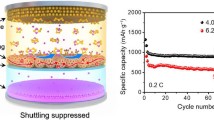
Abstract
Lithium–sulfur (Li–S) battery is one of the new-generation energy storage systems with great potential. However, the development of Li–S battery now is hampered by the shuttle effect and depressed redox kinetics. Herein, we report a composite of Ni3FeN nanoparticles anchored in grid-like porous carbon (PC) spheres as an effective cathode electrocatalyst with simultaneous polysulfide trapping and rapid polysulfides conversion in Li–S battery. The multi-cavity structure of PC with high-efficiency encapsulation ability can significantly improve sulfur utilization and confinement. Furthermore, Ni3FeN nanoparticles embedded in PC cavities render highly active catalytic sites to promote the redox conversion of solvated polysulfide, as revealed by the electrochemical results. Moreover, the catalytic mechanism is further analyzed through density functional theory calculations and in-situ Fourier transform infrared analysis. As a result, PC@Ni3FeN@S cathode delivers an outstanding capacity of 1294 mAh·g−1 at 0.1C and low decay rates of 0.10% per cycle over 500 cycles at 0.5C.
Graphical abstract

摘要
锂硫电池是极具发展潜力的新一代储能系统之一。然而,目前锂硫电池的发展受到了穿梭效应和缓慢氧化还原动力学的限制。在此,我们报道了一种固定在网格状多孔碳球(PC)中的Ni3FeN纳米颗粒复合材料,其作为一种有效的正极电催化剂,可在Li-S电池中同时捕获多硫化物并快速转化多硫化物。碳球中的多腔结构具有高效封装能力,能显著提高硫的利用率并抑制聚硫化物穿梭效应。此外,电化学结果表明,嵌在多孔碳腔中的Ni3FeN纳米颗粒提供了高活性的催化位点,促进了溶剂化多硫化物的氧化还原转化。通过密度泛函理论计算和原位傅里叶变换红外分析进一步分析了催化机理。这种PC@Ni3FeN@S正极在0.1C下提供了1294 mAh·g-1的出色放电比容量,在0.5C下充放电循环500次以上,衰减仅为每圈0.10%。






Similar content being viewed by others
References
Ponnada S, Kiai MS, Gorle DB, Nowduri A. History and recent developments in divergent electrolytes towards high-efficiency lithium–sulfur batteries—a review. Mater Adv. 2021;2:4115. https://doi.org/10.1039/D1MA00332A.
Zhao S, Kang YJ, Liu MJ, Wen BH, Fang Q, Tang YY, He SC, Ma X, Liu MK, Yan Y. Modulating the electronic structure of nanomaterials to enhance polysulfides confinement for advanced lithium–sulfur batteries. J Mater Chem A. 2021;9:18972. https://doi.org/10.1039/D1TA02741G.
Wang ML, Song YZ, Wei N, Shao YL, Sheng G, Sun JY. Universal interface and defect engineering dual-strategy for graphene-oxide heterostructures toward promoted Li–S chemistry. Chem Eng J. 2021;418(15):129407. https://doi.org/10.1016/j.cej.2021.129407.
Xiang HY, Deng NP, Zhao HJ, Wang XX, Wei LY, Wang M, Cheng BW, Kang WM. A review on electronically conducting polymers for lithium–sulfur battery and lithium–selenium battery: progress and prospects. J Energy Chem. 2021;58:523. https://doi.org/10.1016/j.jechem.2020.10.029.
Ng SF, Lau MYL, Ong WJ. Lithium–sulfur battery cathode design: tailoring metal-based nanostructures for robust polysulfide adsorption and catalytic conversion. Adv Mater. 2021. https://doi.org/10.1002/adma.202008654.
Chung S, Manthiram A. Current status and future prospects of metal-sulfur batteries. Adv Mater. 2019;31:1901125. https://doi.org/10.1002/adma.201901125.
Liu T, Tong CJ, Wang B, Liu LM, Zhang S, Lin Z, Wang D, Lu J. Trifunctional electrode additive for high active material content and volumetric lithium-ion electrode densities. Adv Energy Mater. 2019;9:1803390. https://doi.org/10.1002/aenm.201803390.
Feng DC, Kuang JJ, Sun XX, Yuan Q, Yuan ZM, Zhang YH. Research and progress in FeTi based hydrogen storage materials. Chin J Rare Met. 2021;45(3):363. https://doi.org/10.13373/j.cnki.cjrm.XY20010013.
Li G, Wang S, Zhang Y, Li M, Chen Z, Lu J. Revisiting the role of polysulfides in lithium–sulfur batteries. Adv Mater. 2018;30:e1705590. https://doi.org/10.1002/adma.201705590.
Yan YY, Li HT, Cheng C, Yan TR, Gao WP, Mao J, Dai KH, Zhang L. Boosting polysulfide redox conversion of Li–S batteries by one-stepsynthesized Co–Mo bimetallic nitride. J Energy Chem. 2021;61:336. https://doi.org/10.1016/j.jechem.2021.03.041.
Qiu Y, Yin XJ, Wang MX, Li M, Sun X, Jiang B, Zhou H, Tang DY, Zhang Y, Fan LS, Zhang NQ. Constructed conductive CoSe2 nanoarrays as efficient electrocatalyst for high-performance Li–S battery. Rare Met. 2021;40(11):3147. https://doi.org/10.1007/s12598-021-01750-z.
Lin H, Yang L, Jiang X, Li G, Zhang T, Yao Q, Zheng GW, Lee JY. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium–sulfur batteries. Energy Environ Sci. 2017;10:1476. https://doi.org/10.1039/C7EE01047H.
Zhao X, Wu ZS, Dong Y, Lu P, Chen J, Ren W, Cheng HM, Bao X. Free-standing integrated cathode derived from 3D graphene/carbon nanotube aerogels serving as binder-free sulfur host and interlayer for ultrahigh volumetric-energy-density lithiumsulfur batteries. Nano Energy. 2019;60:743. https://doi.org/10.1016/j.nanoen.2019.04.006.
Wang W, Xi K, Li B, Li H, Liu S, Wang J, Zhao H, Li H, Abdelkader AM, Gao X, Li G. A sustainable multipurpose separator directed against the shuttle effect of polysulfides for high-performance lithium–sulfur batteries. Adv Energy Mater. 2022;12:2200160. https://doi.org/10.1002/aenm.202200160.
Wang XL, Li G, Li JD, Zhang YN, Wook A, Yu AP, Chen ZW. Structural and chemical synergistic encapsulation of polysulfides enables ultralong-life lithium–sulfur batteries. Energy Environ Sci. 2016;9:2533. https://doi.org/10.1039/C6EE00194G.
Song Y, Sun Z, Cai J, Wei N, Wang M, Shao Y, Liu Z, Sun J. Accelerated Li–S chemistry at a cooperative interface built in situ. J Mater Chem A. 2019;7:20750. https://doi.org/10.1039/C9TA07342F.
Zhu Q, Xu HF, Shen K, Zhang YZ, Li B, Yang SB. Efficient polysulfides conversion on Mo2CTx MXene for high-performance lithium–sulfur batteries. Rare Met. 2022;41(1):311. https://doi.org/10.1007/s12598-021-01839-5.
Xia G, Zheng Z, Ye J, Li X, Biggs MJ, Hu C. Carbon microspheres with embedded FeP nanoparticles as a cathode electrocatalyst in Li-S batteries. Chem Eng J. 2021;406(15):126823. https://doi.org/10.1016/j.cej.2020.126823.
Eftekhari A, Kim DW. Cathode materials for lithium–sulfur batteries: a practical perspective. J Mater Chem A. 2017;5:17734. https://doi.org/10.1039/C7TA00799J.
Ye Z, Jiang Y, Feng T, Wang Z, Li L, Wu F, Chen R. Curbing polysulfide shuttling by synergistic engineering layer composed of supported Sn4P3 nanodots electrocatalyst in lithium–sulfur batteries. Nano Energy. 2020;70:104532. https://doi.org/10.1016/j.nanoen.2020.104532.
Zhang Z, Luo D, Li G, Gao R, Li M, Li S, Zhao L, Dou H, Wen G, Sy S, Hu Y, Li J, Yu A, Chen Z. Tantalum-based electrocatalyst for polysulfide catalysis and retention for high-performance lithium–sulfur batteries. Matter. 2020;3:920. https://doi.org/10.1016/j.matt.2020.06.002.
Han XR, Guo XT, Xu MJ, Pang H, Ma YW. Clean utilization of palm kernel shell: sustainable and naturally heteroatom-doped porous activated carbon for lithium–sulfur batteries. Rare Met. 2020;39(9):1099. https://doi.org/10.1007/s12598-020-01439-9.
Li Y, Guo XT, Zhang ST, Pang H. Promoting performance of lithium–sulfur battery via in situ sulfur reduced graphite oxide coating. Rare Met. 2021;40(2):417. https://doi.org/10.1007/s12598-020-01498-y.
Zhang J, Liu D, Hou S, Du L, Song H, Liao S, Xue M. Design of a multispherical cavity carbon with in situ silica modifications and its self-humidification application on fuel cell anode support. Adv Mater Interfaces. 2018;5:1800314. https://doi.org/10.1002/admi.201800314.
Xie F, Xie R, Zhang JX, Jiang HF, Du L, Zhang M. Direct reductive quinolyl β-C–H alkylation by multispherical cavity carbon-supported cobalt oxide nanocatalysts. ACS Catal. 2017;7:4780. https://doi.org/10.1021/acscatal.7b01337.
Dai C, Hu L, Li X, Xu Q, Wang R, Liu H, Chen H, Bao SJ, Chen Y, Henkelman G, Li CM, Xu M. Chinese knot-like electrode design for advanced Li–S batteries. Nano Energy. 2018;53:354. https://doi.org/10.1016/j.nanoen.2018.08.065.
Jia X, Zhao Y, Chen G, Shang L, Shi R, Kang X, Waterhouse GIN, Wu LZ, Tung CH, Zhang T. Ni3FeN nanoparticles derived from ultrathin nife-layered double hydroxide nanosheets: an efficient overall water splitting electrocatalyst. Adv Energy Mater. 2016;6:1502585. https://doi.org/10.1002/aenm.201502585.
Li Y, Xu P, Chen G, Mou J, Xue S, Li K, Zheng F, Dong Q, Hu J, Yang C, Liu M. Enhancing Li-S redox kinetics by fabrication of a three dimensional Co/CoP@nitrogen-doped carbon electrocatalyst. Chem Eng J. 2020;380:122595. https://doi.org/10.1016/j.cej.2019.122595.
Ruan S, Huang Z, Cai W, Ma C, Liu X, Wang J, Qiao W, Ling L. Enabling rapid polysulfide conversion kinetics by using functionalized carbon nanosheets as metal-free electrocatalysts in durable lithium–sulfur batteries. Chem Eng J. 2020;385:123840. https://doi.org/10.1016/j.cej.2019.123840.
Wang P, Xi B, Huang M, Chen W, Feng J, Xiong S. Emerging catalysts to promote kinetics of lithium–sulfur batteries. Adv Energy Mater. 2021;11:2002893. https://doi.org/10.1002/aenm.202002893.
Wang Q, Shang L, Shi R, Zhang X, Waterhouse GIN, Wu LZ, Tung CH, Zhang T. 3D carbon nanoframe scaffold-immobilized Ni3FeN nanoparticle electrocatalysts for rechargeable zinc-air batteries’ cathodes. Nano Energy. 2017;40:382. https://doi.org/10.1016/j.nanoen.2017.08.040.
Zhang Y, Ouyang B, Xu J, Jia G, Chen S, Rawat RS, Fan HJ. Rapid synthesis of cobalt nitride nanowires: highly efficient and low-cost catalysts for oxygen evolution. Angew Chem Int Ed. 2016;55:8670. https://doi.org/10.1002/anie.201604372.
Zhang B, Xiao C, Xie S, Liang J, Chen X, Tang Y. Iron–nickel nitride nanostructures in situ grown on surface-redox-etching nickel foam: efficient and ultrasustainable electrocatalysts for overall water splitting. Chem Mater. 2016;28:6931. https://doi.org/10.1021/acs.chemmater.6b02610.
Cao BF, Veith GM, Neuefeind JC, Adzic RR, Khalifah PG. Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction. J Am Chem Soc. 2013;135(51):19186. https://doi.org/10.1021/ja4081056.
Xu K, Chen P, Li X, Tong Y, Ding H, Wu X, Chu WS, Peng Z, Wu C, Xie Y. Metallic nickel nitride nanosheets realizing enhanced electrochemical water oxidation. J Am Chem Soc. 2015;137:4119. https://doi.org/10.1021/ja5119495.
Shalom M, Ressnig D, Yang X, Clavel G, Fellinger TP, Antonietti M. Nickel nitride as an efficient electrocatalyst for water splitting. J Mater Chem A. 2015;3:8171. https://doi.org/10.1039/C5TA00078E.
Zhao M, Peng HJ, Zhang ZW, Li BQ, Chen X, Xie J, Chen X, Wei JY, Zhang Q, Huang JQ. Activating inert metallic compounds for high-rate lithium–sulfur batteries through in situ etching of extrinsic metal. Angew Chem Int Ed. 2019;58:3779. https://doi.org/10.1002/anie.201812062.
Sun Q, He B, Zhang XQ, Lu AH. Engineering of hollow core-shell interlinked carbon spheres for highly stable lithium–sulfur batteries. ACS Nano. 2015;9:8504. https://doi.org/10.1021/acsnano.5b03488.
Greeley J, Jaramillo TF, Bonde J, Chorkendorff I, NøRskov JK. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat Mater. 2006;5:909. https://doi.org/10.1038/nmat1752.
Zhang LH, He B, Li WC, Lu AH. Surface free energy-induced assembly to the synthesis of grid-like multicavity carbon spheres with high level in-cavity encapsulation for lithium–sulfur cathode. Adv Energy Mater. 2017;7:1701518. https://doi.org/10.1002/aenm.201701518.
Qian L, Lu Z, Xu T, Wu X, Tian Y, Li Y, Huo Z, Sun X, Duan X. Trinary layered double hydroxides as high-performance bifunctional materials for oxygen electrocatalysis. Adv Energy Mater. 2015;5:1500245. https://doi.org/10.1002/aenm.201500245.
Ma LB, Zhang WJ, Wang L, Hu Y, Zhu GY, Wang YR, Chen RP, Chen T, Tie ZX, Liu J, Jin Z. Strong capillarity, chemisorption, and electrocatalytic capability of crisscrossed nanostraws enabled flexible, high-rate, and long-cycling lithium sulfur batteries. ACS Nano. 2018;12:4868. https://doi.org/10.1021/acsnano.8b01763.
Ye Z, Wang F, Jia C, Mu K, Yu M, Lv Y, Shao Z. Nitrogen and oxygen-codoped carbon nanospheres for excellent specific capacitance and cyclic stability supercapacitor electrodes. Chem Eng J. 2017;330:1166. https://doi.org/10.1016/j.cej.2017.08.070.
Yuan H, Peng HJ, Li BQ, Xie J, Kong L, Zhao M, Chen X, Huang JQ, Zhang Q. Conductive and catalytic triple-phase interfaces enabling uniform nucleation in high-rate lithium–sulfur batteries. Adv Energy Mater. 2019;9:1802768. https://doi.org/10.1002/aenm.201802768.
Wang Y, Xie C, Liu D, Huang X, Huo J, Wang S. Nanoparticle-stacked porous nickel-iron nitride nanosheet: a highly efficient bifunctional electrocatalyst for overall water splitting. ACS Appl Mater Interfaces. 2016;8:18652. https://doi.org/10.1021/acsami.6b05811.
Zhang SS. Liquid electrolyte lithium/sulfur battery: fundamental chemistry, problems, and solutions. J Power Sources. 2013;231:153. https://doi.org/10.1016/j.jpowsour.2012.12.102.
Yang JL, Zhao SX, Lu YM, Zeng XT, Lv W, Cao GZ. In-situ topochemical nitridation derivative MoO2-Mo2N binary nanobelts as multifunctional interlayer for fast-kinetic Li-sulfur batteries. Nano Energy. 2020;68:104356. https://doi.org/10.1016/j.nanoen.2019.104356.
Liu S, Li J, Yan X, Su Q, Lu Y, Qiu J, Wang Z, Lin X, Huang J, Liu R. Superhierarchical cobalt-embedded nitrogen-doped porous carbon nanosheets as two-in-one hosts for high-performance lithium–sulfur batteries. Adv Mater. 2018;30:1706895. https://doi.org/10.1002/adma.201706895.
Salem HA, Babu G, Rao CV, Arava LMR. Electrocatalytic polysulfide traps for controlling redox shuttle process of Li–S batteries. J Am Chem Soc. 2015;137:11542. https://doi.org/10.1021/jacs.5b04472.
Yu Q, Lu Y, Luo R, Liu X, Huo K, Kim JK, He J, Luo Y. In situ formation of copper-based hosts embedded within 3D N-doped hierarchically porous carbon networks for ultralong cycle lithium–sulfur batteries. Adv Funct Mater. 2018;28:1804520. https://doi.org/10.1002/adfm.201804520.
Dreissig I, Machill S, Salzer R, Krafft C. Quantification of brain lipids by FTIR spectroscopy and partial least squares regression. Spectrochim Acta A Mol Biomol Spectrosc. 2009;71:2069. https://doi.org/10.1016/j.saa.2008.08.008.
Zhou X, Liu TT, Zhao GF, Yang XF, Guo H. Cooperative catalytic interface accelerates redox kinetics of sulfur species for high-performance Li-S batteries. Energy Stor Mater. 2021;40:139. https://doi.org/10.1016/j.ensm.2021.05.009.
Du Z, Chen X, Hu W, Chuang C, Xie S, Hu A, Yan W, Kong X, Wu X, Ji H. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium–sulfur batteries. J Am Chem Soc. 2019;141:3977. https://doi.org/10.1021/jacs.8b12973.
Acknowledgements
The authors acknowledge the financial support provided by the National Natural Science Foundation of China (No. 52064049), the Key National Natural Science Foundation of Yunnan Province (No. 2019FY003023), the International Joint Research Center for Advanced Energy Materials of Yunnan Province (No. 202003AE140001), the Key Laboratory of Solid State Ions for Green Energy of Yunnan University (No. 2019), and the Analysis and Measurements Center of Yunnan University for the sample testing service.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, SM., Li, HN., Zhao, GF. et al. Ni3FeN anchored on porous carbon as electrocatalyst for advanced Li–S batteries. Rare Met. 42, 515–524 (2023). https://doi.org/10.1007/s12598-022-02140-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02140-9