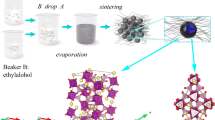
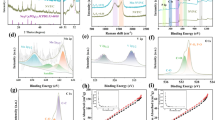
Abstract
Based on the excellent sodium ion mobility of sodium superionic conductor structures, Na3V2(PO4)3 materials have become promising cathode materials in sodium-ion batteries (SIBs). However, inadequate electronic transport of Na3V2(PO4)3 limits the cycling stability and rate performances in SIBs. In this work, high-performance conductive carbon-coated Na3V2(PO4)3 materials are obtained via a simple and facile ball-milling assisted solid-state method by utilizing citric acid as carbon sources. The carbon-coated composite electrodes display a high initial specific capacity of 111.6 mAh·g−1, and the specific capacity could retention reach 92.83% after 100 cycles at 1C with the high coulombic efficiency (99.95%). More importantly, the capacity of conductive carbon-coated nano-sized Na3V2(PO4)3 can remain 48.5 mAh·g−1 at 10C after 3000 cycles (initial capacity of 101.2 mAh·g−1). At the same time, high coulombic efficiency (near 100%) has little decay even at a high rate of 20C during 1000 cycles, demonstrating the excellent cycling stability and remarkable rate performances, and showing potential in large-scale productions and applications.
摘要
基于钠超离子导体结构的优异钠离子迁移速率, Na3V2(PO4)3材料已成为钠离子电池 (SIBs) 中极有发展前景的正极材料。然而, Na3V2(PO4)3的导电性不佳会限制钠离子电池的循环稳定性和速率性能。在这项工作中, 利用柠檬酸作为碳源, 通过一种简单而又容易的球磨辅助固相法, 制备了高性能导电碳包覆的Na3V2(PO4)3材料。碳包覆复合电极的初始比容量高, 为111.6 mAh•g-1。在1C的电流密度下, 循环100次后, 容量保持率达到92.83%, 库仑效率高达99.95%。更重要的是, 在10C下经过3000次循环后, 导电碳涂覆纳米级Na3V2(PO4)3的容量仍可保持48.5 mAh•g-1 (初始容量为101.2 mAh•g-1)。同时, 即使在20C的高倍率下循环1000次, 高库仑效率 (接近100%) 也几乎没有衰减, 证明该材料具有优异的循环稳定性和出色的倍率性能, 并在大规模生产和应用中展现出潜力。
Graphic abstract







Similar content being viewed by others
References
Dunn B, Kamath H, Tarascon JM. Electrical energy storage for the grid: a battery of choices. Science. 2011;334(6058):928.
Hu M, Pang X, Zhou Z. Recent progress in high-voltage lithium ion batteries. J Power Sources. 2013;237:229.
Zou H, Gratz E, Apelian D, Wang Y. A novel method to recycle mixed cathode materials for lithium ion batteries. Green Chem. 2013;15(5):1183.
Nithya C, Gopukumar S. Sodium ion batteries: a newer electrochemical storage. Wiley Interdiscip Rev Energy Environ. 2015;4(3):253.
Barpanda P, Oyama G, Nishimura S, Chung S, Yamada A. A 3.8 V earth-abundant sodium battery electrode. Nat Commun. 2014;5:4358.
Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-Gonzlez J, Rojo T. Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ Sci. 2012;5(3):5884.
Sun Y, Guo S, Zhou H. Exploration of advanced electrode materials for rechargeable sodium-ion batteries. Adv Energy Mater. 2019;9(23):1800212.
Fang C, Huang Y, Zhang W, Han J, Deng Z, Cao Y, Yang H. Routes to high energy cathodes of sodium-ion batteries. Adv Energy Mater. 2016;6(5):1501727.
Chen S, Wu C, Shen L, Zhu C, Huang Y, Xi K, Maier J, Yu Y. Challenges and perspectives for NASICON-type electrode materials for advanced sodium-ion batteries. Adv Mater. 2017;29(48):1700431.
Fang Y, Zhang J, Xiao L, Ai X, Cao Y, Yang H. Phosphate framework electrode materials for sodium ion batteries. Adv Sci. 2017;4(5):1600392.
Goodenough JB, Hong H, Kafalas J. Fast Na+-ion transport in skeleton structures. Mater Res Bull. 1976;11(2):203.
Li G, Jiang D, Wang H, Lan X, Zhong H, Jiang Y. Glucose-assisted synthesis of Na3V2(PO4)3/C composite as an electrode material for high-performance sodium-ion batteries. J Power Sources. 2014;265:325.
Ling R, Cai S, Xie D, Li X, Wang M, Lin Y, Jiang S, Shen K, Xiong K, Sun X. Three-dimensional hierarchical porous Na3V2(PO4)3/C structure with high rate capability and cycling stability for sodium-ion batteries. Chem Eng J. 2018;353:264.
Li H, Bi X, Bai Y, Yuan Y, Shahbazian-Yassar R, Wu C, Wu F, Lu J, Amine K. High-rate, durable sodium-ion battery cathode enabled by carbon-coated micro-sized Na3V2(PO4)3 particles with interconnected vertical nanowalls. Adv Mater Interfaces. 2016;3(9):1500740.
Wang D, Chen N, Li M, Wang C, Ehrenberg H, Bie X, Wei Y, Chen G, Du F. Na3V2(PO4)3/C composite as the intercalation-type anode material for sodium-ion batteries with superior rate capability and long-cycle life. J Mater Chem A. 2015;3:8636.
Didwal PN, Verma R, Min C, Park C. Synthesis of 3-dimensional interconnected porous Na3V2(PO4)3@C composite as a high-performance dual electrode for Na-ion batteries. J Power Sources. 2019;413:1.
Chen H, Huang Y, Mao G, Tong H, Yu W, Zheng J, Ding Z. Reduced graphene oxide decorated Na3V2(PO4)3 microspheres as cathode material with advanced sodium storage performance. Front Chem. 2018;6:174.
Du G, Wang S, Zheng M. 3D CNT decorated Na3V2(PO4)3/C microsphere with outstanding sodium storage performance for Na-ion batteries. Solid State Ion. 2018;317:229.
Ni Q, Bai Y, Li Y, Ling L, Li L, Chen G, Wang Z, Ren H, Wu F, Wu C. 3D electronic channels wrapped large-sized Na3V2(PO4)3 as flexible electrode for sodium-ion batteries. Small. 2018;14(43):1702864.
Nie P, Zhu Y, Shen L, Pang G, Xu G, Dong S, Dou H, Zhang X. From biomolecule to Na3V2(PO4)3/nitrogen-decorated carbon hybrids: highly reversible cathodes for sodium-ion batteries. J Mater Chem A. 2014;2(43):18606.
Wang H, Jiang D, Zhang Y, Li G, Lan X, Zhong H, Zhang Z, Jiang Y. Self-combustion synthesis of Na3V2(PO4)3 nanoparticles coated with carbon shell as cathode materials for sodium-ion batteries. Electrochim Acta. 2015;155:23.
Zheng L, Xue Y, Liu B, Zhou Y, Hao S, Wang Z. High performance Na3V2(PO4)3 cathode prepared by a facile solution evaporation method for sodium-ion batteries. Ceram Int. 2017;43(6):4950.
Fang J, Wang S, Li Z, Chen H, Xia L, Ding L, Wang H. Porous Na3V2(PO4)3@C nanoparticles enwrapped in three-dimensional graphene for high performance sodium-ion batteries. J Mater Chem A. 2016;4(4):1180.
Chen L, Zhao Y, Liu S, Zhao L. Hard carbon wrapped Na3V2(PO4)3@C porous composite extending cycling lifespan for sodium-ion batteries. ACS Appl Mater Interfaces. 2017;9(51):44485.
Venkatesha NJ, Ramesh S. Citric acid-assisted synthesis of nanoparticle copper catalyst supported on an oxide system for the reduction of furfural to furfuryl alcohol in the vapor phase. Ind Eng Chem Res. 2018;57(5):1506.
Li S, Ge P, Zhang C, Sun W, Hou H, Ji X. The electrochemical exploration of double carbon-wrapped Na3V2(PO4)3: towards long-time cycling and superior rate sodium-ion battery cathode. J Power Sources. 2017;366:249.
Song W, Ji X, Wu Z, Zhu Y, Yang Y, Chen J, Jing M, Li F, Banks CE. First exploration of Na-ion migration pathways in the NASICON structure Na3V2(PO4)3. J Mater Chem A. 2014;2(15):5358.
Sui YL, Wu L, Hong W, Liu JQ, Zhang XP, Li W, Zhong SK. Synthesis and electrochemical properties of spherically shaped LiVPO4F/C cathode material by a spray drying-roasting method. Rare Met. 2019. https://doi.org/10.1007/s12598-019-01340-0.
Xu CC, Wang Y, Li L, Wang YJ, Jiao LF, Yuan HT. Hydrothermal synthesis mechanism and electrochemical performance of LiMn0.6Fe0.4PO4 cathode material. Rare Met. 2019;38(1):29.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2017YFB0102000), Major Program of the National Natural Science Foundation of China (No. 51890865) and the State Key Program of National Natural Science of China (No. 61835014).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, D., Cai, P., Zou, GQ. et al. Ultra-stable carbon-coated sodium vanadium phosphate as cathode material for sodium-ion battery. Rare Met. 41, 115–124 (2022). https://doi.org/10.1007/s12598-021-01743-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01743-y