Abstract
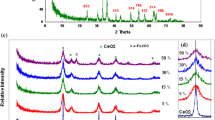
A series of nanosized CeO2–Fe2O3 mixed-oxide nanocomposites with different Ce4+/Fe3+ molar ratios were successfully prepared by a co-precipitation technique. The surface area increased with Fe2O3 content increasing up to 60 wt% in the composite. However, with further increase in Fe2O3 content, the surface area began to decrease. The reduction processes of the CeO2–Fe2O3 nanocomposites were studied in a hydrogen atmosphere at 300–600 °C. The reduction rates increased by increasing both the temperature and Fe2O3 content in the nanocomposites. The microstructure of the reduced composites at 500 °C illustrated the presence of a considerable number of macro- and micro-pores. The activation energy values were calculated which were in the range of 3.56–5.37 kJ·mol−1 at the initial stages (up to 35% reduction) and 5.21–10.2 kJ·mol−1 at the final stages (up to 80% reduction) of reduction. The rate-controlling mechanisms in both the initial and final reduction stages were determined, and the initial reaction stage was controlled by combined gaseous diffusion and interfacial chemical reaction mechanisms for all the composites except for pure CeO2, which was controlled by a chemical reaction mechanism. The final reaction stage was controlled by a gaseous diffusion mechanism for some composites, while for the others it was controlled by combined gaseous diffusion and interfacial chemical reaction mechanisms. The hydrogen sorption properties of the nanocomposites were studied by pressure composition isotherms using a volumetric method. Hydrogen storage in the nanocomposites increased by increasing the temperature because of the formation of oxygen vacancies which enhance atomic H adsorption and function as strong adsorption sites forming more metal hydride covalent bonds.









Similar content being viewed by others
References
Bao H, Chen X, Fang J, Jiang Z, Huang W. Structure-activity relation of Fe2O3–CeO2 composite catalysts in CO oxidation. Catal Lett. 2008;125(1–2):160.
Wei Y. Syngas generation from methane using a chemical-looping concept: a review of oxygen carriers. J Chem. 2013. https://doi.org/10.1155/2013/294817.
Xiao S, Mei H, Han D, Dassios KG, Cheng L. Ultralight lamellar amorphous carbon foam nanostructured by SiC nanowires for tunable electromagnetic wave absorption. Carbon. 2017;122:718.
Mei H, Zhao X, Bai S, Li Q, Xia J, Bai H, Cheng L. Tuning SERS properties of pattern-based MWNTs-AuNPs substrates by adjustment of the pattern spacings. Carbon. 2018;136:38.
Zhu X, Sun L, Zheng Y, Wang H, Wei Y, Li K. CeO2 modified Fe2O3 for the chemical hydrogen storage and production via cyclic water splitting. Int J Hydrogen Energy. 2014;39(25):13381.
Channei D, Inceesungvorn B, Wetchakun N, Phanichphant S, Nakaruk A, Koshy P, Sorrell C. Photocatalytic activity under visible light of Fe-doped CeO2 nanoparticles synthesized by flame spray pyrolysis. Ceram Int. 2013;39(3):3129.
Channei D, Inceesungvorn B, Wetchakun N, Phanichphant S. Kinetics study of photocatalytic activity of flame-made unloaded and Fe-loaded CeO2 nanoparticles. Int J Photoenergy. 2013. https://doi.org/10.1155/2013/484831.
Arul NS, Mangalaraj D, Ramachandran R, Grace AN, Han JI. Fabrication of CeO2/Fe2O3 composite nanospindles for enhanced visible light driven photocatalysts and supercapacitor electrodes. J Mater Chem A. 2015;3(29):15248.
Mei H, Huang W, Hua C, Xu Y, Cheng L. A novel approach to strengthen naturally pored wood for highly efficient photodegradation. Carbon. 2018;139:378.
Kanan SM, El-Kadri OM, Abu-Yousef IA, Kanan MC. Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors. 2009;9(10):8158.
Neri G, Bonavita A, Rizzo G, Galvagno S, Capone S, Siciliano P. A study of the catalytic activity and sensitivity to different alcohols of CeO2–Fe2O3 thin films. Sens Actuators B Chem. 2005;111:78.
Liu C, Shan H, Liu L, Li S, Li H. High sensing properties of Ce-doped α-Fe2O3 nanotubes to acetone. Ceram Int. 2014;40(1):2395.
Al-Kelesh H, Halim KA, Nasr M. Synthesis of heavy tungsten alloys via powder reduction technique. J Mater Res. 2016;31(19):2977.
Kang KS, Kim CH, Cho WC, Bae KK, Woo SW, Park CS. Reduction characteristics of CuFe2O4 and Fe3O4 by methane; CuFe2O4 as an oxidant for two-step thermochemical methane reforming. Int J Hydrogen Energy. 2008;33(17):4560.
El-khalek NAA, Naser MI, Yassin KE, Al-Kelesh H. Studying the reduction behavior of eastern desert iron after benefication using falcon concentrator. J Ore Dress. 2014;16(31):11.
Halim KA. Isothermal reduction behavior of Fe2O3/MnO composite materials with solid carbon. Mater Sci Eng A. 2007;452:15.
Llusar M, Royo V, Badenes J, Tena M, Monrós G. Nanocomposite Fe2O3–SiO2 inclusion pigments from post-functionalized mesoporous silicas. J Eur Ceram Soc. 2009;29(16):3319.
Mohapatra M, Anand S. Synthesis and applications of nano-structured iron oxides/hydroxides—a review. Int J Eng Sci Technol. 2010;2(8):127.
Dharanipragada NA, Meledina M, Galvita VV, Poelman H, Turner S, Van Tendeloo G, Detavernier C, Marin GB. Deactivation study of Fe2O3–CeO2 during redox cycles for CO Production from CO2. Ind Eng Chem Res. 2016;55(20):5911.
Turkdogan E, Vinters J, Vinters J. Gaseous reduction of iron oxides: part I. Reduction of hematite in hydrogen. Metall Mater Trans B. 1971;2(11):3175.
Turkdogan E, Olsson R, Vinters J. Gaseous reduction of iron oxides: part II. Pore characteristics of iron reduced from hematite in hydrogen. Metall Mater Trans B. 1971;2(11):3189.
El-Geassy AA, Shehata KA, Ezz SY. Mechanism of iron-oxide reduction with hydrogen-carbonmonoxide mixtures. Trans Iron Steel Inst Jpn. 1977;17(11):629.
El-Geassy A. Gaseous reduction of Fe2O3 compacts at 600 to 1050 °C. J Mater Sci. 1986;21(11):3889.
Gold R, Sandall W, Cheplick P, MacRae D. Plasma reduction of iron oxide with H and natural gas at 100 kW and 1 MW. Ironmak Steelmak. 1977;4(1):10.
Pan F, Zhang J, Chen HL, Su YH, Kuo CL, Su YH, Chen SH, Lin KJ, Hsieh PH, Hwang WS. Effects of rare earth metals on steel microstructures. Materials. 2016;9(6):417.
Maisang W, Phuruangrat A, Thongtem S, Thongtem T. Photoluminescence and photonic absorbance of Ce2(MoO4)3 nanocrystal synthesized by microwave–hydrothermal/solvothermal method. Rare Met. 2018;37(10):868.
El Rouby W, Farghali A, Hamdedein A. Microwave synthesis of pure and doped cerium (IV) oxide (CeO2) nanoparticles for methylene blue degradation. Water Sci Technol. 2016;74(10):2325.
Farghali AA, El Rouby WM, Hamdedein A. Effect of hydrothermal conditions on microstructures of pure and doped CeO2 nanoparticles and their photo-catalytic activity: degradation mechanism and pathway of methylene blue dye. Res Chem Intermed. 2017;43(12):7171.
Yu XX, Sun J, Li ZT, Dai H, Fang HJ, Zhao JF, Yin DF. Solidification behavior and elimination of undissolved Al2CuMg phase during homogenization in Ce-modified Al–Zn–Mg–Cu alloy. Rare Met. 2018. https://doi.org/10.1007/s12598-018-1172-1.
Aboud AA, Al-Kelesh H, El Rouby WM, Farghali AA, Hamdedein A, Khedr MH. CO2 responses based on pure and doped CeO2 nano-pellets. J Mater Res Technol. 2018;7(1):14.
Cao S, Shi M, Wang H, Yu F, Weng X, Liu Y, Wu Z. A two-stage Ce/TiO2–Cu/CeO2 catalyst with separated catalytic functions for deep catalytic combustion of CH2Cl2. Chem Eng J. 2016;290:147.
Shi Z, Yang P, Tao F, Zhou R. New insight into the structure of CeO2–TiO2 mixed oxides and their excellent catalytic performances for 1,2-dichloroethane oxidation. Chem Eng J. 2016;295:99.
Aresta M, Dibenedetto A, Pastore C, Cuocci C, Aresta B, Cometa S, De Giglio E. Cerium (IV) oxide modification by inclusion of a hetero-atom: a strategy for producing efficient and robust nano-catalysts for methanol carboxylation. Catal Today. 2008;137(1):125.
Lin S, Su G, Zheng M, Ji D, Jia M, Liu Y. Synthesis of flower-like Co3O4–CeO2 composite oxide and its application to catalytic degradation of 1,2,4-trichlorobenzene. Appl Catal B. 2012;123:440.
Wang W, Zhu Q, Dai Q, Wang X. Fe doped CeO2 nanosheets for catalytic oxidation of 1,2-dichloroethane: effect of preparation method. Chem Eng J. 2017;307:1037.
Sahoo S, Mohapatra M, Pandey B, Verma H, Das R, Anand S. Preparation and characterization of α-Fe2O3–CeO2 composite. Mater Charact. 2009;60(5):425.
Brito PC, Santos DA, Duque JGS, Macêdo MA. Structural and magnetic study of Fe-doped CeO2. Physica B. 2010;405(7):1821.
Cardillo D, Konstantinov K, Devers T. The effects of cerium doping on the size, morphology, and optical properties of α-hematite nanoparticles for ultraviolet filtration. Mater Res Bull. 2013;48(11):4521.
Janoš P, Kuráň P, Pilařová V, Trögl J, Šťastný M, Pelant O, Henych J, Bakardjieva S, Životský O, Kormunda M. Magnetically separable reactive sorbent based on the CeO2/γ-Fe2O3 composite and its utilization for rapid degradation of the organophosphate pesticide parathion methyl and certain nerve agents. Chem Eng J. 2015;262:747.
Arena F, Gumina B, Lombardo AF, Espro C, Patti A, Spadaro L, Spiccia L. Nanostructured MnOx catalysts in the liquid phase selective oxidation of benzyl alcohol with oxygen: part I. Effects of Ce and Fe addition on structure and reactivity. Appl Catal B. 2015;162:260.
Gobara HM, Aboutaleb WA, Hashem KM, Hassan SA, Henein SA. A novel route for synthesis of α-Fe2O3–CeO2 nanocomposites for ethanol conversion. Mater Sci. 2017;52(1):550.
Broom DP. Hydrogen sorption properties of materials. Hydrogen Storage Mater. 2011. https://doi.org/10.1007/978-0-85729-221-6.
Prabhukhot Prachi R, Wagh Mahesh M, Gangal Aneesh C. A review on solid state hydrogen storage material. Adv Energy Power. 2016;4(2):11.
Lee DH, Cha KS, Lee YS, Kang KS, Park CS, Kim YH. Effects of CeO2 additive on redox characteristics of Fe-based mixed oxide mediums for storage and production of hydrogen. Int J Hydrogen Energy. 2009;34(3):1417.
Santos MC, Kesler O, Reddy ALM. Nanomaterials for energy conversion and storage. J Nanomater. 2012. https://doi.org/10.1155/2012/159249.
Wang G, Yang Y, Han D, Li Y. Oxygen defective metal oxides for energy conversion and storage. Nano Today. 2017;13:23.
Gu Z, Li K, Qing S, Zhu X, Wei Y, Li Y, Wang H. Enhanced reducibility and redox stability of Fe2O3 in the presence of CeO2 nanoparticles. RSC Adv. 2014;4(88):47191.
Galvita VV, Poelman H, Bliznuk V, Detavernier C, Marin GB. CeO2-Modified Fe2O3 for CO2 utilization via chemical looping. Ind Eng Chem Res. 2013;52(25):8416.
Li K, Haneda M, Gu Z, Wang H, Ozawa M. Modification of CeO2 on the redox property of Fe2O3. Mater Lett. 2013;93:129.
Farghali AA, Sayed SG. Synthesis, characterisation and photo-catalytic activity of CeO2/Fe2O3 nano-composite. Int J Nanopart. 2015;8(2):171.
Kongzhai L, Hua W, Yonggang W, Mingchun L. Preparation and characterization of Ce1−xFexO2 complex oxides and its catalytic activity for methane selective oxidation. J Rare Earths. 2008;26(2):245.
Kurian M, Kunjachan C. Effect of lattice distortion on physical properties and surface morphology of nanoceria framework with incorporation of iron/zirconium. Nano-Struct Nano-Objects. 2015;1:15.
Sohn S, Kim D. Modification of Langmuir isotherm in solution systems—definition and utilization of concentration dependent factor. Chemosphere. 2005;58(1):115.
Khedr MH. Isothermal reduction kinetics at 900–1100 °C of NiFe2O4 sintered at 1000–1200 °C. J Anal Appl Pyrol. 2005;73(1):123.
Szekely J, Evans JW, Sohn HY. Gas–Solid Reactions. New York: Academic Press; 1976.
Ma D, Lu Z, Tang Y, Li T, Tang Z, Yang Z. Effect of lattice strain on the oxygen vacancy formation and hydrogen adsorption at CeO2 (111) surface. Phys Lett A. 2014;378(34):2570.
Liu F, Chen C, Guo H, Saghayezhian M, Wang G, Chen L, Chen W, Zhang J, Plummer E. Unusual Fe–H bonding associated with oxygen vacancies at the (001) surface of Fe3O4. Surf Sci. 2017;655:25.
Zhang C, Geng X, Li J, Luo Y, Lu P. Role of oxygen vacancy in tuning of optical, electrical and NO2 sensing properties of ZnO1−x coatings at room temperature. Sens Actuators B Chem. 2017;248:886.
Tan X, Lan H, Xie H, Zhou G, Jiang Y. Role of surface oxygen species of mesoporous CeCu oxide catalyst in OVOCs catalytic combustion. J Environ Chem Eng. 2017;5(2):2068.
Hai G, Huang J, Cao L, Jie Y, Li J, Wang X, Zhang G. Influence of oxygen deficiency on the synthesis of tungsten oxide and the photocatalytic activity for the removal of organic dye. J Alloys Compd. 2017;690:239.
Acknowledgements
The authors gratefully acknowledge Prof. M. H. Khedr (Materials Science and Nanotechnology Department, Faculty of Postgraduate Studies for Advanced Sciences (PSAS), Beni-Suef University, Egypt) for his contributions. The authors also acknowledge Beni-Suef University for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sayed, S.G., El Rouby, W.M.A. & Farghali, A.A. Preparation and characterization of (CeO2)x–(Fe2O3)1−x nanocomposites: reduction kinetics and hydrogen storage. Rare Met. 39, 218–229 (2020). https://doi.org/10.1007/s12598-019-01244-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-019-01244-z